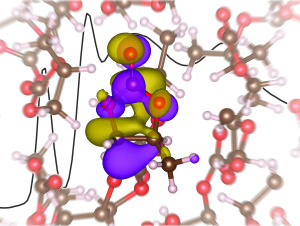
X-ray absorption spectra, interpreted using first-principles electronic structure calculations, provide insight into the solvation of the lithium ion in propylene carbonate.
Image: Rich Saykally, Berkeley Labs
The Electrochemical Society’s Stephen Harris, along with a team of researchers from Berkeley Lab, have found a possible avenue to a better electrolyte for lithium-ion batteries.
Harris – an expert on lithium-ion batteries and chemist at Berkeley Lab’s Materials Science Division – believes that he and his team have unveiled something that could lead to applying lithium-ion batteries to large-scale energy storage.
Researchers around the world know that in order for lithium-ion batteries to store electrical energy for the gird or power electric cars, they must be improved. The team at Berkeley decided to take on this challenge and found surprising results in the first X-ray absorption spectroscopy study of a model lithium electrode, which has provided a better understanding of the liquid electrolyte.
Previous simulations have predicted a tetrahedral solvation structure for the lithium-ion electrolyte, but the new study yields different results.
“Our results indicate a solvation number of 4.5, which points to a non-tetrahedral solvation structure for the lithium ions,” says Harris. “This contradicts numerous theoretical studies which indicated a primarily tetrahedral coordination structure with a solvation number near 2 or 3, depending on the prevalence of ion pairing. Based on our results, to design better performing electrolytes, future computational models will need to move beyond tetrahedral coordination structures.”
Previous researchers trying to address this issue and improve the batteries have focused on the electrodes and solid electrolyte interphase, thereby creating a lack of capabilities for the requisite experiments.
This from Berkeley National Laboratory:
This deficiency was addressed by Saykally and his group with their development of a unique liquid microjet technology in which two aqueous samples rapidly mix and flow through a finely tipped silica nozzle only a few micrometers in diameter. The resulting liquid beam travels a few centimeters in a vacuum chamber before it is intersected by an X-ray beam then collected and condensed out. This liquid microjet system has been set up at Beamline 8.0.1 of Berkeley Lab’s Advanced Light Source (ALS). Beamline 8.0.1 is a high flux undulator beamline that produces X-ray beams optimized for X-ray spectroscopy.
You can read “X-Ray absorption spectroscopy of LiBF4 in propylene carbonate: a model lithium ion battery electrolyte,” in the journal Physical Chemistry Chemical Physics.
Join Harris and other brilliant scientific minds by becoming a member of ECS today!
You can read more of Harris’ work on lithium-ion batteries, including this article entitled “Design of Solid Electrolyte Interphase to Improve Li-Ion Battery Cycle Efficiency,” in ECS’s Digital Library.


