On January 27, 2021, The Electrochemical Society hosted Prof. Christopher Muratore’s live webinar, “Two-dimensional Materials for Scalable Modular Electronic Pathogen Sensors.” Prof. Muratore is the Ohio Research Scholars Endowed Chair Professor in the Chemical and Materials Engineering Department at the University of Dayton, U.S.
Prof. Muratore described the global need to move beyond point-of-care diagnostics to inexpensive, simple-to-use pathogen tests readily available to individuals on a daily basis.
NOTE: Registration is required to view the webinar.
ECS thanks Semilab, the generous sponsor of Prof. Muratore’s webinar.
Q&A
These are Dr. Muratore’s responses to many of the questions participants asked in the Q&A session following his talk.
Q1. Can aptamers be used to functionalize surfaces?
Yes! There are some nice papers from our group and colleagues. Here are a couple of examples of publications describing peptides adhering to molybdenum disulfide (MoS2) surfaces:
- “Interpretable molecular models for molybdenum disulfide and insight into selective peptide recognition”
- “Printed biomolecular templates for 2D material patterning”
Q2. How do sensors react to different strains of the virus?
If the antibody fragment bound to the sensor surface can detect the difference between strains, the sensor devices will also. The sensors are only limited by the ability of antibodies to differentiate between strains.
Q3. The type of laser used to produce MoS2: is it a pulsed nanosecond laser?
We used a continuous wave, 514 nm laser. You can learn more about sensor fabrication via these articles:
- “Laser writing of electronic circuitry in thin film molybdenum disulfide: A transformative manufacturing approach”
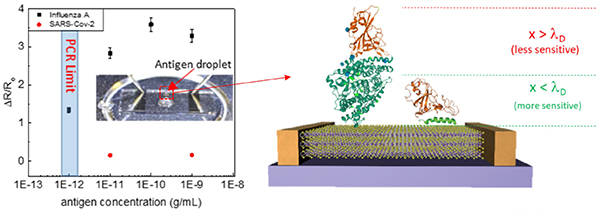
- “Biofunctionalized Two-dimensional MoS2 Receptors for Rapid Response Modular Electronic SARS-CoV-2 and Influenza A Antigen Sensors”
Q4. How is surface functionalization done?
We control sulfur vacancy concentration with the laser annealing conditions. The disulfide hinge region of the antibody fragments has high affinity for this surface.
Q5. A sensing material is more likely to respond to multiple viruses and bio-groups similarly. How is selectivity to COVID-19 realized?
Selectivity to COVID-19 is realized via the attachment of monoclonal antibodies complementary to the desired antigen. The surface becomes selective as only the target antigen will bind.
Q6. Can this technology differentiate various COVID-19 strains?
As in response to Q2, the sensors have the same limitations as our antibodies. If an antibody can detect the difference between strains, the sensor devices will also.
Q7. Why can’t we use electrochemical impedance spectroscopy?
We could, but we are focused on mass production of very inexpensive chips (less than $0.50 each) compatible with a straightforward measurement interface. I think a dc resistance measurement is difficult to top in terms of simplicity.
Q8. What are the effects of layers (monolayer, bilayer, etc.) on detection? The electrical properties of some 2D materials (like PtSe2) are dependent on a number of layers.
You are correct. We have examined different numbers of layers and are focused on five molecular layers as this is the best compromise between sensitivity and ease of synthesis/fabrication.
Q9. Can biomolecular be absorbed on the surface of the 2D materials? Is there any specific bonding between them?
We tailor the biorecognition elements (BREs), whether antibody-based or peptide-based, to adsorb on the MoS2 surface. The antigen itself may indeed bind to the MoS2 directly, but we can ensure a dense layer of BRE to avoid direct interactions with MoS2 surface.
Q10. How close are you to the production of this?
We are working hard on this facet of the work right now, so hopefully by summer 2021.
Learn more upcoming about ECS Webinars.