 The Electrochemical Society hosted Matthew D. L. Garayt’s live webinar, “A Comprehensive Method for Assembly & Design Optimization of Single-Layer Pouch Cells,” on October 23, 2024. A live Question and Answer session followed. Matthew’s answers to some of the questions not addressed during the broadcast follow.
The Electrochemical Society hosted Matthew D. L. Garayt’s live webinar, “A Comprehensive Method for Assembly & Design Optimization of Single-Layer Pouch Cells,” on October 23, 2024. A live Question and Answer session followed. Matthew’s answers to some of the questions not addressed during the broadcast follow.
NOTE: Many of the questions submitted are addressed in “A Guide to Making Highly Reproducible Li-Ion Single-Layer Pouch Cells for Academic Researchers,” the paper Matthew and his colleagues published in the Journal of The Electrochemical Society (JES).
Q&A
Why is there a need for an extra current collector? How might the double-sided phenomenon be different if the current collector is coated on both sides rather than placed on the extra current collector?
Regarding the first part of the question, the extra free-standing current collector is used for flexibility with coating selection. You can alternatively have a single current collector that is cut with a little protrusion of uncoated current collector upon which the tab is welded. However, this typically requires clean edges for the coating, which is not always a guarantee at the lab scale, so the free-standing current collector is used instead.
To address the second part of the question, without the free-standing current collector, the voltage polarization should not increase like it did in the case of the two-sided positive electrode SLP. Apart from that, if this second side is wet with electrolyte and there is a path for Li-ions to diffuse in the electrolyte, it will (de)lithiate Li-ions that will negatively affect performance. We have observed the same phenomenon in coin cells with double-sided electrodes.
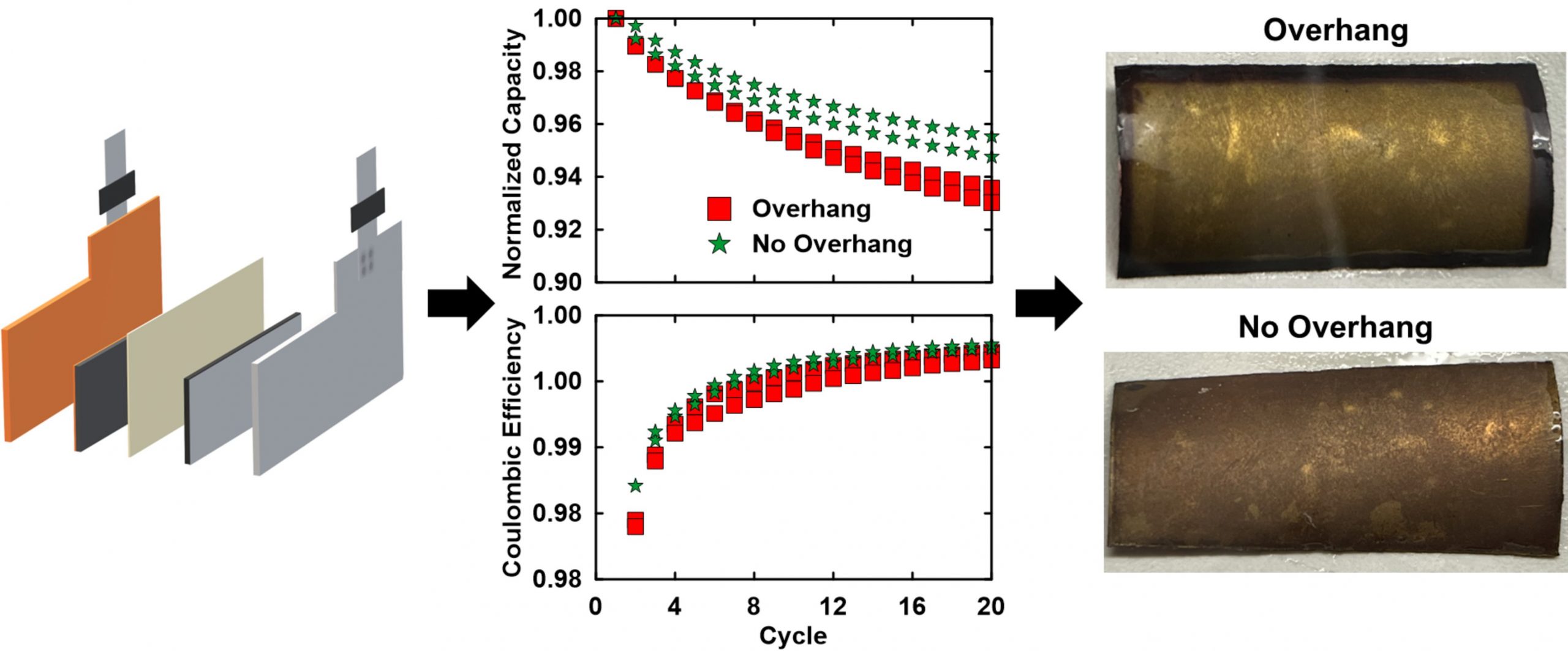
It’s my understanding that having double-sided negative electrodes for the external electrodes is common for industrial pouch cells. Do you think that we would see improvements in capacity retention if they were single-sided, instead, based on your SLP results? Why might manufacturers still use double-sided electrodes for even the outermost layers (i.e., bookends) of the stack?
It is certainly common for commercial manufacturers to only manufacture double-sided coatings as it means the electrode coater’s settings do not need to change to make a single-sided electrode. Typically, the negative electrode is the one that will have an unused second side, which is typically wound in separator and therefore would be wet with electrolyte. This leads to effective loss of Li inventory through Li getting kinetically trapped in these second sides during typical C-rate operation. Therefore, this adversely affects the cycle life of these cells in a way that is proportional to the ratio of unused electrode area to total electrode area. Just like with an overhang, the smaller this ratio, the better for cycle life.
An additional note, if you have access to a high-quality 18650 or 21700 cylindrical cell for disassembly, pay attention to whether the outermost winding of the negative electrode is double- or single-side coated. The best cylindrical cells typically have this section single-side coated, which means that during coating there has to be a regular gap on one side. As mentioned, this introduces challenges in manufacturing but should lead to a better product for the end user.
In a solid state battery which doesn’t have liquid electrolyte, do you think Li diffusion to the back side of coating on the unused second side would still happen?
In the case of a true all solid state cell that uses absolutely no liquid electrolyte, as long as there is no solid electrolyte or direct coating contact between the two sides of the electrode, then Li will not diffuse into the unused side, and it will not have any effect on cycle life. Of course, it will still mean an overall slightly lower cell-level energy density and higher bill of materials cost due to the “wasting” of useable active material.
How do you mitigate the curving of the single-sided electrodes after calendaring?
This is a practical issue for SLPs. If it is possible to decrease the calendaring pressure, then it will relieve the problem; however, this is typically not possible as cell makers want the lowest possible porosity. Therefore, as was shown in the assembly guide for the SLPs, using a small piece of tape on the current collector side of the electrode to hold it in place can help. This is also why having an extra free-standing current collector is beneficial as it can help to keep the electrodes flat. In fact, you can use a thicker free-standing current collector, say ~1.5 mm thick aluminum or steel for the positive or negative, respectively, which helps to keep the electrode coatings from curling.
Do you adjust electrolyte additives to match capacity loading or keep the amount of additive the same?
This is an excellent point. In our testing, we took the typical academic approach which meant we did not change the amount of additives in the electrolyte. However, after a rigorous study for your own electrolyte formulation, it may be better to adjust the additive amount by the electrolyte volume to cell capacity ratio for additives such as vinylene carbonate (VC) that are reduced on the negative electrode and are integral to forming an effective solid-electrolyte interphase (SEI). For a given additive amount, having a larger electrolyte volume to cell capacity ratio means more total additive and could change the performance of the cell, so for the fairest comparison, adjusting the additive amount for the specific cell type is advised depending on what the data suggests. You can go even further with looking into how changing the solvent ratio based on which solvents react more with each electrode or the salt concentration given different electrolyte volume to capacity ratios, but as with many things, it may not be worthwhile.





