For organizations interested in expanding their networks within corporate, academic, governmental, and scientific spheres, ECS offers an advantageous alternative to individual membership. 
Institutional membership with ECS admits your organization into an elite group of scientists, academics, and professionals. It gives your organization access to the vast arrays of information, people, and breaking research that ECS has to offer.
Moreover, institutional membership secures your organization’s place within an ever expanding, collaborative network of innovative thinkers and member organizations.
Want to get a better sense of the scope of this growing network? Check out our list of current institutional ECS members!
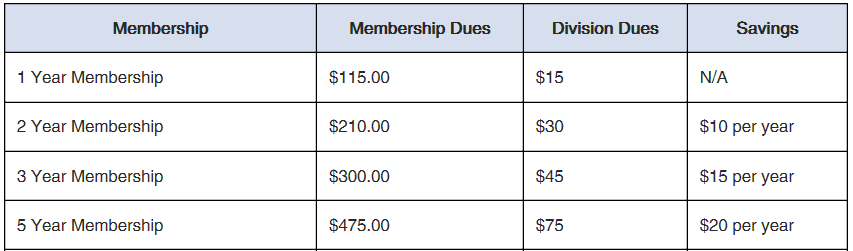
To accommodate the varied needs of prospective institutional member organizations, ECS offers five different levels of institutional membership. Each level has its own distinct set of benefits and discounts. Select the level of institutional membership which best suits your organization!