By: Timothy H. Dixon, University of South Florida
 This summer I worked on the Greenland ice sheet, part of a scientific experiment to study surface melting and its contribution to Greenland’s accelerating ice losses. By virtue of its size, elevation and currently frozen state, Greenland has the potential to cause large and rapid increases to sea level as it melts.
This summer I worked on the Greenland ice sheet, part of a scientific experiment to study surface melting and its contribution to Greenland’s accelerating ice losses. By virtue of its size, elevation and currently frozen state, Greenland has the potential to cause large and rapid increases to sea level as it melts.
When I returned, a nonscientist friend asked me what the research showed about future sea level rise. He was disappointed that I couldn’t say anything definite, since it will take several years to analyze the data. This kind of time lag is common in science, but it can make communicating the issues difficult. That’s especially true for climate change, where decades of data collection may be required to see trends.
A recent draft report on climate change by federal scientists exploits data captured over many decades to assess recent changes, and warns of a dire future if we don’t change our ways. Yet few countries are aggressively reducing their emissions in a way scientists say are needed to avoid the dangers of climate change.
While this lack of progress dismays people, it’s actually understandable. Human beings have evolved to focus on immediate threats. We have a tough time dealing with risks that have time lags of decades or even centuries. As a geoscientist, I’m used to thinking on much longer time scales, but I recognize that most people are not. I see several kinds of time lags associated with climate change debates. It’s important to understand these time lags and how they interact if we hope to make progress.


 Researchers have found a new method for finding lithium, used in the lithium-ion batteries that power modern electronics, in supervolcanic lake deposits.
Researchers have found a new method for finding lithium, used in the lithium-ion batteries that power modern electronics, in supervolcanic lake deposits.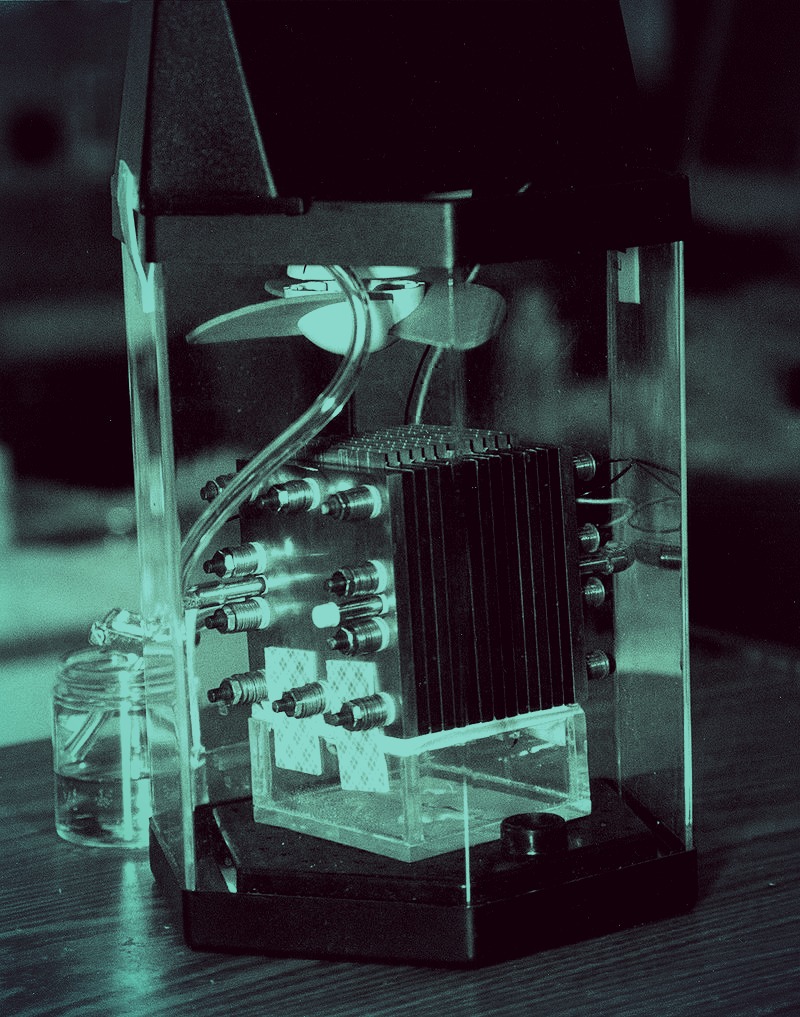

 Researchers from Lappeenranta University of Technology (LUT) and VTT Technical Research Centre of Finland have successfully created food out of electricity and carbon dioxide, which they hope could one day be used to help solve world hunger.
Researchers from Lappeenranta University of Technology (LUT) and VTT Technical Research Centre of Finland have successfully created food out of electricity and carbon dioxide, which they hope could one day be used to help solve world hunger. When will cars powered by gas-guzzling internal combustion engines become obsolete? Not as soon as it seems, even with the latest automotive news out of Europe.
When will cars powered by gas-guzzling internal combustion engines become obsolete? Not as soon as it seems, even with the latest automotive news out of Europe. Scientists have created a single catalyst that could simplify the process of splitting water into hydrogen and oxygen to produce clean energy.
Scientists have created a single catalyst that could simplify the process of splitting water into hydrogen and oxygen to produce clean energy. Scientists have found that a common enzyme can speed up—by 500 times—the rate-limiting part of the chemical reaction that helps the Earth lock away, or sequester, carbon dioxide in the ocean.
Scientists have found that a common enzyme can speed up—by 500 times—the rate-limiting part of the chemical reaction that helps the Earth lock away, or sequester, carbon dioxide in the ocean. The global development of industry, technology, and the transportation sector has resulted in massive consumption of fossil fuels. As these fuels are burned, emissions are released—namely carbon dioxide. According to the U.S. Environmental Protection Agency, combustion of petroleum-based products resulted in
The global development of industry, technology, and the transportation sector has resulted in massive consumption of fossil fuels. As these fuels are burned, emissions are released—namely carbon dioxide. According to the U.S. Environmental Protection Agency, combustion of petroleum-based products resulted in