Image: ReubenGBrewer
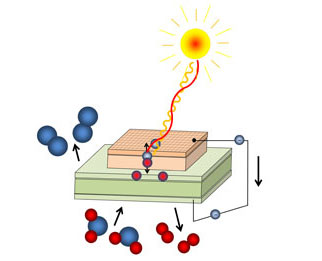
An interdisciplinary team, including 32 year ECS member Stuart Licht and ECS student member Matthew Lefler, has developed a way to make electric vehicles that are not only carbon neutral, but carbon negative – capable of reducing the amount of atmospheric carbon dioxide as they operate by transforming the greenhouse gas.
By replacing the graphite electrodes that are currently being used in the development of lithium-ion batteries for electric cars with carbon materials recovered from the atmosphere, the researchers have been able to develop a recipe for converting collected carbon dioxide into batteries.
This from Vanderbilt University:
The team adapted a solar-powered process that converts carbon dioxide into carbon so that it produces carbon nanotubes and demonstrated that the nanotubes can be incorporated into both lithium-ion batteries like those used in electric vehicles and electronic devices and low-cost sodium-ion batteries under development for large-scale applications, such as the electric grid.
The research is not the first time scientists have shown progress in collecting and converting harmful greenhouse gases from the environment.
Typically, carbon dioxide conversion revolves around transforming the gas into low-value fuels such as methanol. These conversions often do not justify the costs.
(MORE: Read “Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes.“)
However, the new process produces better batteries that are not only expected to be efficient, but also cost effective.



 The U.S. Department of Energy recently released a new series of posters illuminating a new generation of sustainable energy and green jobs. The series is reminiscent of the famous imagery created for the Works Progress Administration, only this time, the images depict a renewable energy revolution.
The U.S. Department of Energy recently released a new series of posters illuminating a new generation of sustainable energy and green jobs. The series is reminiscent of the famous imagery created for the Works Progress Administration, only this time, the images depict a renewable energy revolution.