A new water-based air-conditioning system cools air to as low as 18 degrees Celsius (about 64 degrees Fahrenheit) without using energy-intensive compressors and environmentally harmful chemical refrigerants.
This technology could potentially replace the century-old air-cooling principle that is still used in modern-day air-conditioners. Suitable for both indoor and outdoor use, the new system is portable and can be customized for all types of weather conditions.
The team’s novel air-conditioning system is cost-effective to produce, and it is also more eco-friendly and sustainable.


 Incorporating organic electronic materials in the field of bioelectronics has indicated promising potential in interfacing with biological systems, including neuroscience applications. Researchers from Linköping University are taking a major step forward in that work with their development of the world’s first complementary electrochemical logic circuits that can function for long periods of time in water.
Incorporating organic electronic materials in the field of bioelectronics has indicated promising potential in interfacing with biological systems, including neuroscience applications. Researchers from Linköping University are taking a major step forward in that work with their development of the world’s first complementary electrochemical logic circuits that can function for long periods of time in water. A team of researchers from the University of Toronto is looking to give wasted materials new value by developing a new catalyst that could help recycle carbon dioxide into plastic.
A team of researchers from the University of Toronto is looking to give wasted materials new value by developing a new catalyst that could help recycle carbon dioxide into plastic.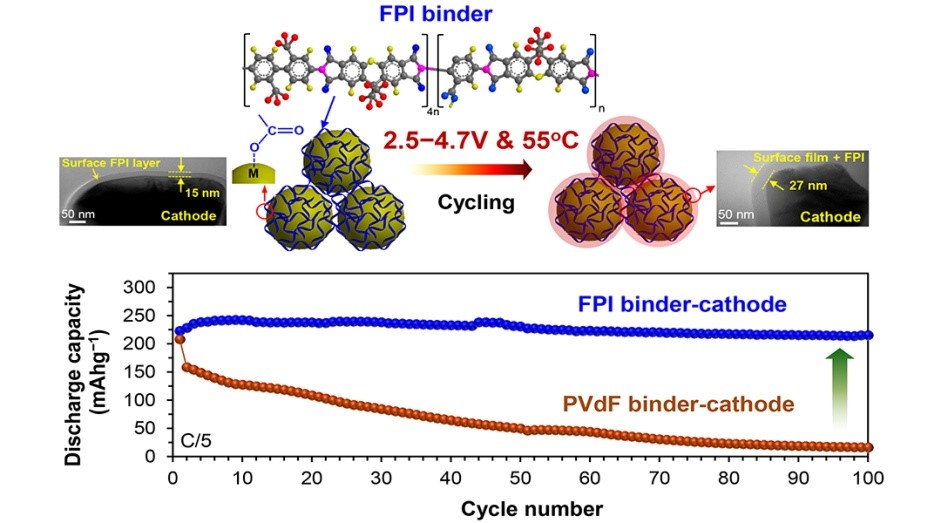
 Do you know of an organization that is hiring? Are you connected with recruiters or managers looking to fill positions? You now have a unique opportunity to help ECS work towards engaging employers through our ECS Career Expo.
Do you know of an organization that is hiring? Are you connected with recruiters or managers looking to fill positions? You now have a unique opportunity to help ECS work towards engaging employers through our ECS Career Expo. Extended deadline: February 12, 2018
Extended deadline: February 12, 2018 The 2018 Society elections are upon us and ECS wants you to learn more about the candidates from the candidates.
The 2018 Society elections are upon us and ECS wants you to learn more about the candidates from the candidates. Extended Deadline: February 16, 2018
Extended Deadline: February 16, 2018 Join us as ECS and SMEQ comes together for the
Join us as ECS and SMEQ comes together for the