Reginald Penner (pictured) and doctoral candidate developed a nanowire-based batter that can be charged hundreds of thousands of times.
Image: Daniel A. Anderson/UC Irvine
Researchers at the University of California, Irvine may have just developed the ever-lasting battery.
A recent study, published in ACS Energy Letters, details a nanowire-based battery material that can be recharged hundreds of thousands of times – making more realistic the idea of a battery that would never need to be replaced.
Potential applications for the battery range from computers and smartphones to cars and spacecrafts.
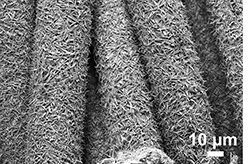
Highly-conductive nanowires have always been thought appropriate for battery design, but were held back by the fact that their fragility causes them to breakdown after multiple charging cycles. By coating a gold nanowire in a manganese dioxide shell and encasing the assembly in an electrolyte, the researchers have turn the frail structure into something that has almost infinite recharging capabilities.
Mya Le Thai, a doctoral candidate, led the charge on the research – cycling the tested electrode up to 200,000 times over a three month period without loss of capacity or damage to the nanowire.
“Mya was playing around, and she coated this whole thing with a very thin gel layer and started to cycle it. She discovered that just by using this gel, she could cycle it hundreds of thousands of times without losing any capacity,” said Reginald M. Penner, chair of UC Irvine’s chemistry department and ECS member. “That was crazy, because these things typically die in dramatic fashion after 5,000 or 6,000 or 7,000 cycles at most.”
Thai believes that this study shows that nanowire-based batteries could be commercially viable, and potentially the next big break in battery technology.