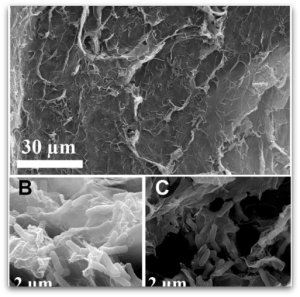
Graphene is called the “wonder material” with good reason. The material hosts a slew of unique chemical and physical properties, with applications from fuel cells to biomedical to energy storage.
Now, a team from MIT is taking the material and applying it to infrared sensors to create next-gen night vision goggles. Additionally, the team is looking to take that same technology and apply it to high-tech windshields and smartphones.
We achieve night vision capabilities through thermal imaging that allows people to see otherwise invisible infrared rays that are shed as heat. This technology is useful for many different applications, such as assisting soldiers and firefighters in their duty. While night vision devices currently exist, they need bulky cooling systems to create a useful image.
Because of graphene’s electrical qualities, researchers have known that the material would be an excellent infrared detector. The team at MIT took this idea and moved forwarding in creating a less bulky night vision goggle through the utilization of graphene.