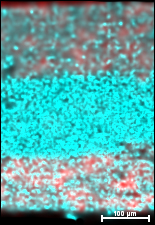
This new development will lead to accelerated improvements in the materials’ uniformity, stability, and efficiency.
Source: University of Washington
In light of the growth in solar energy research, scientists have been directing a lot of attention toward perovskites. The materials’ wide range of use and potential to outpace silicon-based semiconductors in the field of solar cells makes perovskites an interesting area of research with great potential.
Researchers from the University of Washington, in conjunction with the University of Oxford, have discovered a new quality to perovskites that could help engineer a better solar cell.
The researchers have shown in their research that, contrast to popular belief, the perovskites are uniform in composition. The materials actually contain flaws that can be engineered to improve solar devices even further.
“In that short amount of time, the ability of these materials to convert sunlight directly into electricity is approaching that of today’s silicon-based solar cells, rivaling technology that took 50 years to develop,” said Dane deQuilettes, a University of Washington doctoral student. “But we also suspect there is room for improvement.”