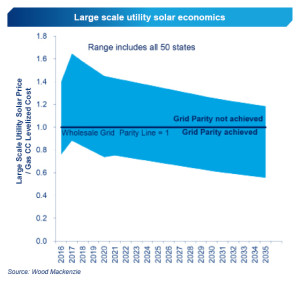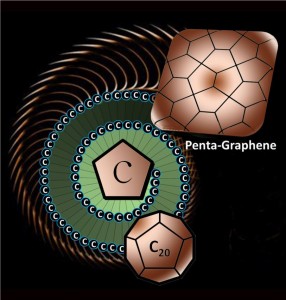
The newly discovered material, called penta-graphene, is a single layer of carbon pentagons that resembles the Cairo tiling, and that appears to be dynamically, thermally and mechanically stable.
Image: VCU
Researchers from Virginia Commonwealth University (VCU) in conjunction with universities in China and Japan have discovered a new structural variant of carbon that they are coining “penta-graphene.”
The new material is comprised of a very thin sheet of pure carbon that is especially unique due to its exclusively pentagonal pattern. Thus far, the penta-graphene appears to be dynamically, thermally and mechanically stable.
“The three last important forms of carbon that have been discovered were fullerene, the nanotube and graphene. Each one of them has unique structure. Penta-graphene will belong in that category,” said the paper’s senior author and distinguished professor in the Department of Physics at VCU, Puru Jena in a press release.
The inspiration for this new development came from the pattern of the tiles found paving the streets of Cairo. Professor at Peking University and adjunct professor at VCU, Qian Wang, got the inspiration that inevitably led to penta-graphene while dining in Beijing.