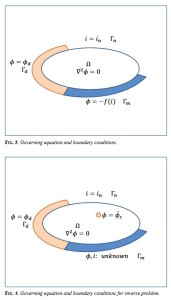
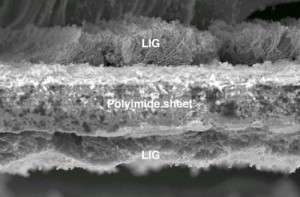
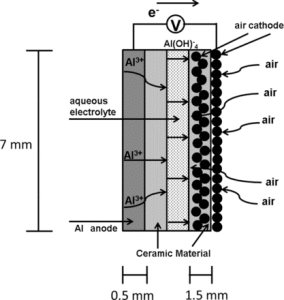
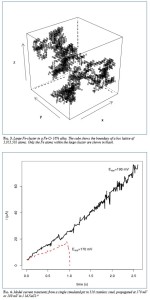
 An article by N.J. Laycock, D.P. Krouse, S.C. Hendy, and D.E. Williams published in the latest issue of Interface.
An article by N.J. Laycock, D.P. Krouse, S.C. Hendy, and D.E. Williams published in the latest issue of Interface.
Stainless steels and other corrosion resistant alloys are generally protected from the environment by ultra-thin layers of surface oxides, also called passive films. Unfortunately, these films are not perfect and their Achilles’ heel is a propensity to catastrophic local breakdown, which leads to rapid corrosion of the metallic substructure. Aside from the safety and environmental hazards associated with these events, the economic impact is enormous.
In the oil and gas and petrochemical industries, it is of course usually possible to select from experience a corrosion-resistant alloy that will perform acceptably in a given service environment. This knowledge is to a large extent captured in industry or company-specific standards, such as Norsok M1.
However, these selections are typically very conservative because the limits tend to be driven by particular incidents or test results, rather than by fundamental understanding. Decision-making can be very challenging, especially in today’s mega-facilities, where the cost of production downtime is often staggeringly large. Thus significant practical benefits could be gained from reliable quantitative models for pitting corrosion of stainless steels. There have been several attempts to develop purely stochastic models of pitting corrosion.
Read the rest.