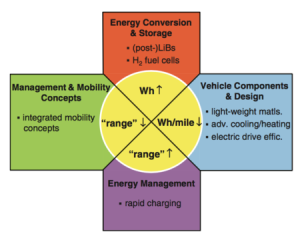
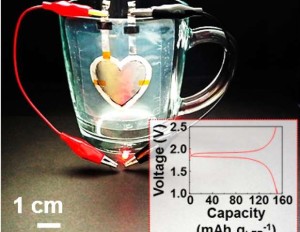
Researchers across the globe have been investing more and more effort into developing new materials to power the next generation of devices. With the population growing and energy demands rising, the need for smaller, faster, and more efficient batteries is more prevalent than ever.
While some researchers are attempting to develop complex material combinations to tackle this issue, researchers from Rice University are going back to basics by developing a clay-based electrolyte.
Utilizing clay as a primary material in a lithium ion battery could address current issues that the battery has with high temperature performance. With clay, the researchers were able to supply stable electrical power in environments with temperatures up 120°C. The addition of clay to the electrode could allow lithium ion batteries to function in harsh environments including space, defense, and oil and gas applications.