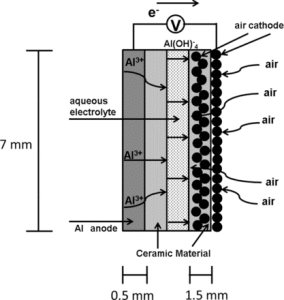
You can thank “dendrites” when your smartphone battery goes from a solid 40 percent charge to completely dead in a matter of 20 minutes. Thankfully, researchers out of Purdue University are researching these dendrites – otherwise known as the slayer of lithium-ion batteries – and developing something that could greatly improve the li-ion.
Dendrites work to destroy lithium-ion batteries by forming an anode electrode and growing until they affect battery performance – potentially resulting in complete battery failure.
The new study out of Purdue University explores this issue with the intention of creating a safer and longer-lasting lithium-ion battery that could be charged within minutes instead of hours.