 New technology that mimics the branches and leaves of a cottonwood tree can generate electricity with the help of the wind.
New technology that mimics the branches and leaves of a cottonwood tree can generate electricity with the help of the wind.
Researchers say that the new technology is not meant to be a replacement for wind turbines, but could offer an alternative electricity source for those looking for small, unobtrusive machines to transform wind into energy.
“The possible advantages here are aesthetics and its smaller scale, which may allow off-grid energy harvesting,” says Michael McCloskey, co-author of the study. “We set out to answer the question of whether you can get useful amounts of electrical power out of something that looks like a plant. The answer is ‘possibly,’ but the idea will require further development.”
On top of efficiency and affordability, consumers are also looking for alternative energy technologies to be aesthetically attractive, as demonstrated in Tesla’s solar roof.
According to McCloskey, cell phone towers in urban locations are sometimes camouflaged as trees to offer better aesthetic properties. The researchers believe that towers such as this, which already host fake leaves, could be greatly improved by implementing this technology to tap energy from the leaves and provide further functionality.


 When a May 2016 crash
When a May 2016 crash  The
The 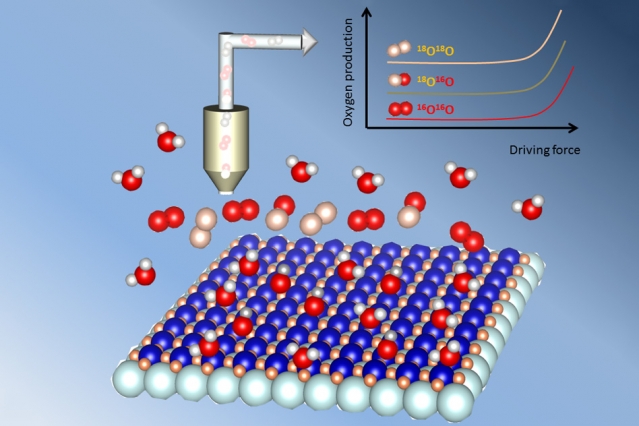
 Biofuels have become a promising potential alternative for traditional fossil fuels. However, producing biofules only make sense if the greenhouse gasses emitted are less than other means of producing energy.
Biofuels have become a promising potential alternative for traditional fossil fuels. However, producing biofules only make sense if the greenhouse gasses emitted are less than other means of producing energy. Renewable energy is on the rise, but how we store that energy is still up for debate.
Renewable energy is on the rise, but how we store that energy is still up for debate. Bill Gates is taking climate change head on with his newly formed
Bill Gates is taking climate change head on with his newly formed  A team of researchers at Case Western Reserve University is building a flow battery prototype to provide cleaner, cheaper power.
A team of researchers at Case Western Reserve University is building a flow battery prototype to provide cleaner, cheaper power. Just over ten years ago, the number of electric vehicles on the road could be counted in the hundreds. Now, more than
Just over ten years ago, the number of electric vehicles on the road could be counted in the hundreds. Now, more than