The Electrochemical Society hosted Evan Wenbo Zhao’s live webinar, “Operando NMR Methods for Redox Flow Batteries and Ammonia Synthesis,” on November 13, 2024. A live Question and Answer session followed. Evan’s answers to some of the questions not addressed during the broadcast are below.
The Electrochemical Society hosted Evan Wenbo Zhao’s live webinar, “Operando NMR Methods for Redox Flow Batteries and Ammonia Synthesis,” on November 13, 2024. A live Question and Answer session followed. Evan’s answers to some of the questions not addressed during the broadcast are below.
NOTE: Registration is required to view the webinar replay.
Q&A
Can we imagine an application of NMR characterization methods for other applications like supercapacitor degradation?
Yes; (in situ) NMR has been applied to study supercapacitors, mainly on charge storage mechanisms. Here is a classic paper from Prof. Clare Grey’s group at Cambridge University: https://pubs.acs.org/doi/10.1021/ja410287s.
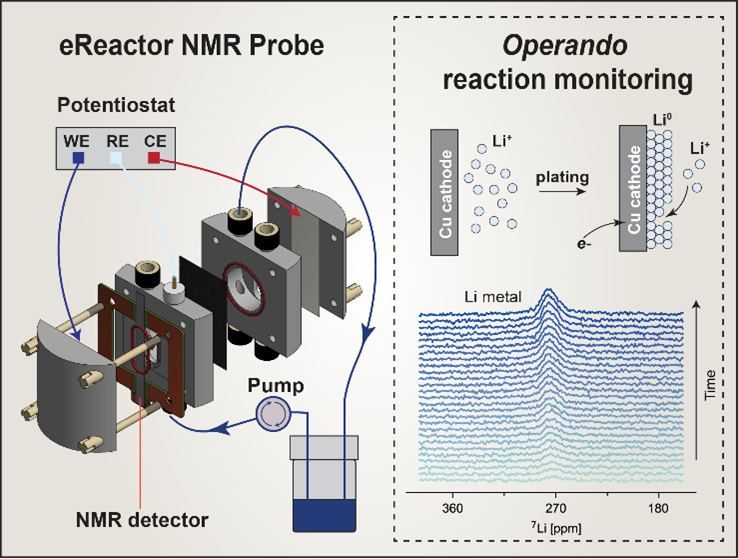
I am very interested in how we connect the system with NMR to study dimerization and what type of isomers we obtain during reaction. I think we need a session on how the connection with the flow cell is set up.
We will publish a paper on the technical details of the setup soon. Stay tuned.
Can the system of NMR connected to the flow cell be used for carbon dioxide reduction? We know that the products depends on CO2 solubility on the medium and whether or not we are going to obtain mono or di acids for example.
Yes, it can. We have studied CO2 reduction via inline NMR in the past two years, and a publication will be out soon. In a nutshell, we can quantify liquid products, carbonate species, water crossover, and pH changes.
If bubbles are generated during a reaction, how can this affect the NMR characterization?
Bubbles are detrimental to NMR measurement because they distort the magnetic field to an extent that the spectral resolution is lost.
In other battery systems like SIBs, did in situ XRD results agree with NMR for the prediction of sodium-ion storage in the hard carbon?
I am not an expert in sodium-ion batteries. This paper probably answers your question: https://pubs.rsc.org/en/content/articlelanding/2016/cc/c6cc06990h.
Is water in the mixture with the anthraquinone or is it run separately as a reference to confirm if radicals are being generated?
Water is the solvent and therefore mixed with anthraquinones.
Is the performance of a benchtop EPR comparable with the classical full-scale instrument?
In my experience, the benchtop EPR is close enough to the classical X-band system, at least for studying dissolved organic radical species.
I am very interested in your experimental setup in slide 16. Could you share more details about that? For example, what’s the material of the tubing? Will the flow rate or something like the length of the tubing inside the detection chamber influence the signal detection? What are the biggest challenges for designing an operando electrochemical cell like in slide 29 and how to overcome them? My last question: is it possible to realize in situ observation to electrocatalysts?
We are going to publish the detailed design of the coupled NMR and EPR benchtop method very soon, as well as the cell design on slide 29. Yes, it is possible to perform in situ NMR on electrocatalysts; for example, the Li-based catalyst for ammonia synthesis or Pt-based electrocatalyst. In my opinion, in situ NMR for studying electrocatalysis has been under exploited, compared to other characterization methods. We have a mini-review on this topic: https://www.sciencedirect.com/science/article/pii/S2772516224000032.
NMR is a bulk technique, so how can it be used to study interfaces in RFB?
I would not call NMR just a bulk technique. Indeed, it is more straightforward to study bulk, but also possible to study interfaces. Our eReactor NMR probe is designed to study interfaces by confining the RF field to the interface on a length scale of 100 μm (see https://www.sciencedirect.com/science/article/pii/S1090780724000508). Another technique called dynamic nuclear polarization is also useful for studying interfaces in batteries (see https://www.sciencedirect.com/science/article/abs/pii/S0926204021000515).
Please explain the difference between in situ and operando?
Operando refers to the measurement performed on an operating (electrochemical) device. In situ refers to the measurement performed on an intact device while the device can be operating or not.
Have you calculated from 7Li NMR spectra the fraction of Li metal formed or consumed during reactions?
We have not done it yet, but it is possible.
Using in situ/operando, can you estimate the reversibility of processes (get quantitative information); what is challenging in that?
Yes, we can. NMR is quantitative, so by quantifying the amount of active or degradation species, the reversibility of the processes is quantified.
How should I—or can I—distinguish between lithium plating and lithium corrosion applying operando NMR? Signals have similar chemical shifts.
It is based on the Li metal signal intensity and the electrochemical condition. During electrochemical reduction, Li metal is plated as the Li metal signal increases, likely accompanied by corrosion. During resting, Li corrosion occurs, reflected by the decreased Li metal signal.
How do you perform solid state NMR? You detected solid lithium forming in the cell for the ammonia synthesis topic, but do you apply MAS technique? And is the solid lithium on the electrodes?
The measurement was performed without MAS. Due to the Knight shift, metallic Li has a characteristic chemical shift approximately 260 ppm away from the diamagnetic Li region at 0-10 ppm, convenient for detection. Yes, the solid lithium is formed on the electrode, but we cannot rule out the possibility of subsequent formation of “dead” lithium detached from the electrode.
Is it possible to study changes in the solid state electrode with the operando NMR methodology?
Yes, it is possible.
Did your group try to investigate the corrosion product of THF upon contacting Li metal? Also, would you be able to see other Li species on 7Li NMR, or will they just stay around 0 ppm?
Yes, we have data on the THF corrosion product, which we are still analyzing. Yes, we can see other Li species via 7Li or 6Li NMR which have different chemical shifts. Also, solution Li species have sharper signals while solid Li species have broader signals, so line shapes also help.
Can you see LiN2H and LiNH2 intermediates in 7Li NMR?
Yes, we can, via ex situ magic angle spinning solid state NMR.
Do you foresee any issues investigating changes in the lithium mediated nitrogen reduction electrolyte in line, in the same way as for redox flow batteries?
The inline technique for studying redox flow battery is transferable to studying electrolyte in lithium mediated nitrogen reduction.