
Jie Xiao
Photo credit: Andrea Starr | Pacific Northwest National Laboratory
On November 11, 2020, Jie Xiao, Laboratory Fellow and Group Leader of the Battery Materials & System Group, Pacific Northwest National Laboratory, and Arkansas Research Alliance Scholar & Associate Professor, Inorganic Chemistry, University of Arkansas, presented “Electrochemistry in Rechargeable Lithium Metal Batteries” via a live webinar presentation hosted by The Electrochemical Society.
Xiao’s talk covered the electrochemistry underlying Li metal dendrite growth; pouch cell level understanding of Li dendrites and their implications in cell performance; and bridged the scientific gap between academic research and industry needs.
View Dr. Xiao’s webinar presentation
Note: Registration is required to view the webinar.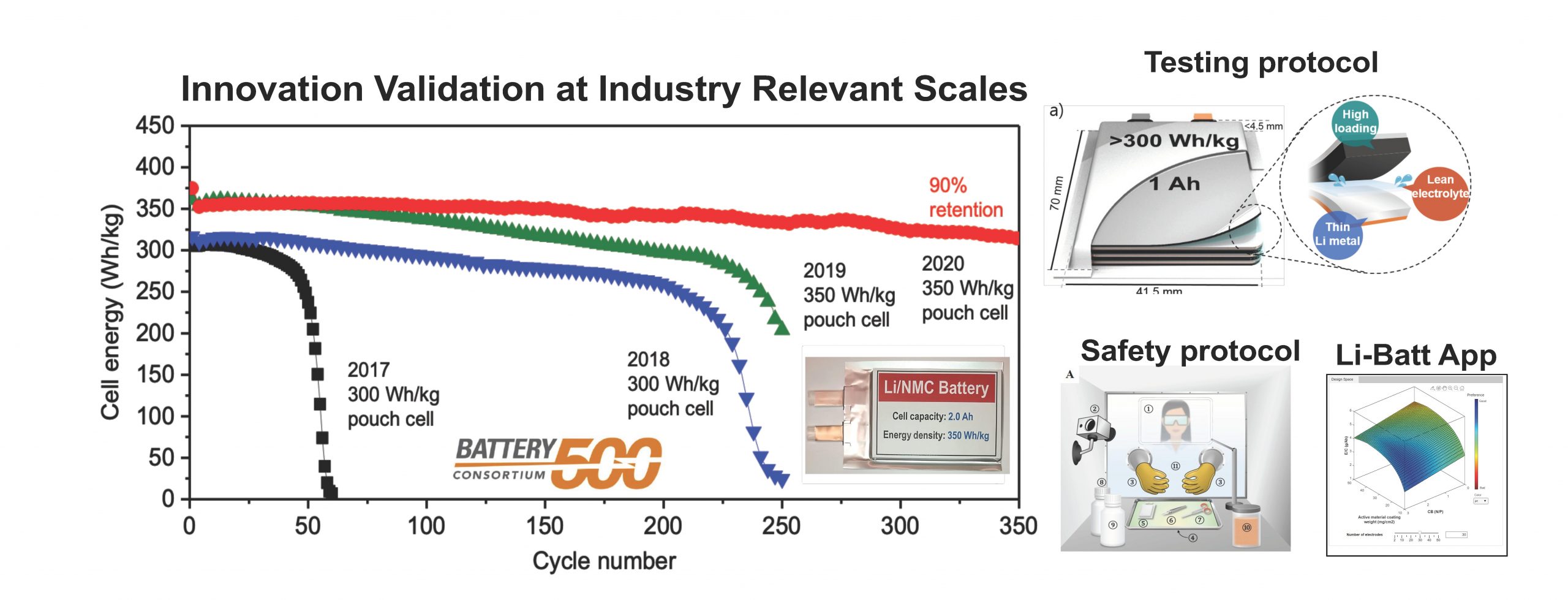
ECS thanks the generous sponsors of Dr. Xiao’s webinar: Hiden Analytical, Biologic, The Royal Society of Chemistry, and Admiral Instruments.
Don’t miss ECS’s next webinar at 1000h ET on December 12 when Dr. Dan Steingart presents “Without Sound and Fury, but Signifying Something: Learnings from Acoustic Approaches for Battery Characterization.” Follow this link for information about all past and pending ECS webinars.
Q&A
Attendees had the opportunity to ask Dr. Xiao questions in a Q&A session following the talk. Here are responses to many of the questions posed.
Q: DME is a volatile solvent; what about the safety of these LMBs?
A: For traditional “safe” electrolytes such as carbonate solvent based ones, they are not compatible with lithium metal leading to very limited cycling, a few tens of cycles in realistic Li metal batteries. For the “good” electrolytes that stabilize Li metal cycling, they have not been fully assessed by safety testing. However, it is important that we understand the fundamentals of why Li metal has limited cycling first and then step further into enhancing the safety step-by-step. If the Li metal batteries only last for 10-50 cycles in safe electrolytes, they still cannot be used broadly.
Q: Based on slide 9 data, does it follow high temperatures? There is less dendritic growth because conduction is faster in high temperatures?
A: It will be better from the ion transport point of view. However, side reactions also become more aggressive at elevated temperatures. So an optimized temperature will be needed.
Q: If you double areal capacity (maybe by increasing electrode thickness), would a pouch cell need a smaller number of layers? How would this change pack level analysis?
A: Our Li-Batt app considers all different possible combinations to make Li metal pouch cells with desired cell level energy. Please refer to the Li-Batt Design App.
Q: How do the new generation electrolytes help prevent TM dissolution at high charged states?
A: The extensively cycled cathode shows little or no degradation at least for the first few hundreds of cycles. The limiting factor of Li metal batteries from the first few hundred cyclings is still Li metal and interface not cathode or TM dissolution. Please refer to our previously published work on the cathode impact.
Q: As mentioned before, slower charge means slow Lithium moving to the Lithium metal forms concentrating Lithium at one place which results in shorting. Why charge at a slower rate?
A: To clarify, slower charge means slower electrochemical reduction since you are tuning the current, not stirring the electrolyte. The Li+ diffusion rate in liquid electrolyte does not change but they’re being reduced much slower on the interface. So you have more Li+ ions nearby and thus milder concentration gradient.
Q: Many papers discussed using different materials as lithium hosts. Do you think this kind of method is practical?
A: A host structure reduces the real current density which equals to reduce charge rate so it has some positive effects. However, host structures are porous and will absorb a large amount of electrolyte, increasing the parasitic weight of the entire cell. Li/host ratio needs to at least be higher than LiC6 (lithiated graphite) otherwise it is difficult to bring any gains. Tabbing of those host structures is another challenge. Similar coin cell protocol is needed when testing these host structures. That is lean electrolyte, thick cathode and balanced Li.
Q: Usually, very concentrated electrolytes have slow diffusivities which leads to stronger gradients and lower ionic conductivity. Are there limits to max salt concentration?
A: Concentrated electrolyte is very difficult to fully wet thick and dense electrodes. That is why PNNL is working on localized concentrated electrolyte. The molarity is less than about 1.5M if you check our published work.
Q: You mentioned if there is a slow ion transport leads to a strong concentration gradient in the bulk. But at fast charging, isn’t the fast ion transport leads to a higher concentration gradient, which leads to lithium plating in LIBs?
A: Fast charging means higher current density/over potential to drive the electrochemical reaction not faster Li+ transport in the electrolyte. Please refer to my published article in Science last year for more details.
Q: It is common to use slow charge and fast discharge in LMBs. Do they perform worse in slow charge/slow discharge scenarios?
A: Fast discharge is because of the power requirement when using batteries. Slow charge is for the reason I mentioned during webinar. In general slow charge/discharge benefits electrochemical reactions because of lower over potential and less side reaction in addition to the concentration gradient issues.
Q: Please explain if the surface coating on Li metal will suppress the dendrite growth?
A: Eventually the thin coating layers will be interweaved with porous Li. It is unknown to me how all the newly exposed Li surfaces can be protected still.
Q: What are the relative impedance changes in the cells upon cycling?
A: It will increase eventually due to SEI formation but it’s not linear because the surface area of Li is also increasing.
Q: Is ML-batt Can ML-Assisted Li-Batt design be applied to lithium-sulfur batteries?
A: Yes, it includes NMC(111-811), LFP, S, CFx or custom designed cathode if you have input the parameters by hand.
Q: I’m curious about the “lean electrolyte” condition. Is it still enough to fill all the porous electrode pores, or some pores stay empty?
A: Yes, as you can tell from the capacity based on cathode.
Q: Will a lithium metal anode system still use the copper current collector?
A: Yes, this works.
Q: You mentioned the ratio of electrolyte to electrode material in pouch cells is much less than that in coin cells. How much does the evaporation of electrolyte contribute to the pouch cell’s degradation over cycles?
A: The pouch cell is well sealed and there is no evaporation issue over cycling.


