The Electrochemical Society hosted Arumugam Manthiram’s live webinar, “Sustainable Next-generation Battery Chemistries,” on September 20, 2023. Dr. Manthiram took audience questions during a live Question and Answer session at the end of the presentation. He kindly answered in writing questions not answered during the broadcast. His responses follow.
View Dr. Manthiram’s WebinarNOTE: Registration is required to view the webinar.
Q&A
Why has it taken three decades to employ high-nickel cathodes in lithium ion batteries?
It is hard to stabilize Ni3+ under normal synthesis conditions at high temperatures, unlike Co3+, which leads to Ni2+ formation and high cation mixing in LiNiO2 structure. Now we have learnt to synthesize LiNiO2 with high oxygen flow at moderate temperatures to stabilize Ni3+ and suppress lithium volatilization and Ni2+ formation. This has enabled us to increase the Ni content in layered oxide cathodes. However, high-nickel oxides still suffer from high surface reactivity with the electrolyte.
What is the major roadblock to using lithium nickel oxide cathode?
Even though we can synthesize LiNiO2 with little cation disorder, it is hampered by high surface reactivity with the electrolyte and formation of resistive spinel-like and rock salt phases and a thick cathode-electrolyte interphase (CEI), resulting in capacity fade. See a paper my group published in Joule on October 8 for more information: https://www.cell.com/joule/pdf/S2542-4351(23)00396-3.pdf
Why are we not able to use layered oxide cathodes with cheaper ions like iron?
Fe3+ with five d electrons and a high-spin configuration in an octahedral site has three electrons in the low-lying t2g band and two electrons in the upper-lying eg band (a symmetric distribution of electrons among the five d orbitals). This leads to zero crystal field stabilization energy in both octahedral and tetrahedral sites, so an equal stabilization of Fe3+ in both octahedral and tetrahedral sites, which results in an easy migration of Fe3+ from octahedral to tetrahedral sites, and in an easy layered to spinel transition that causes voltage and capacity decay.
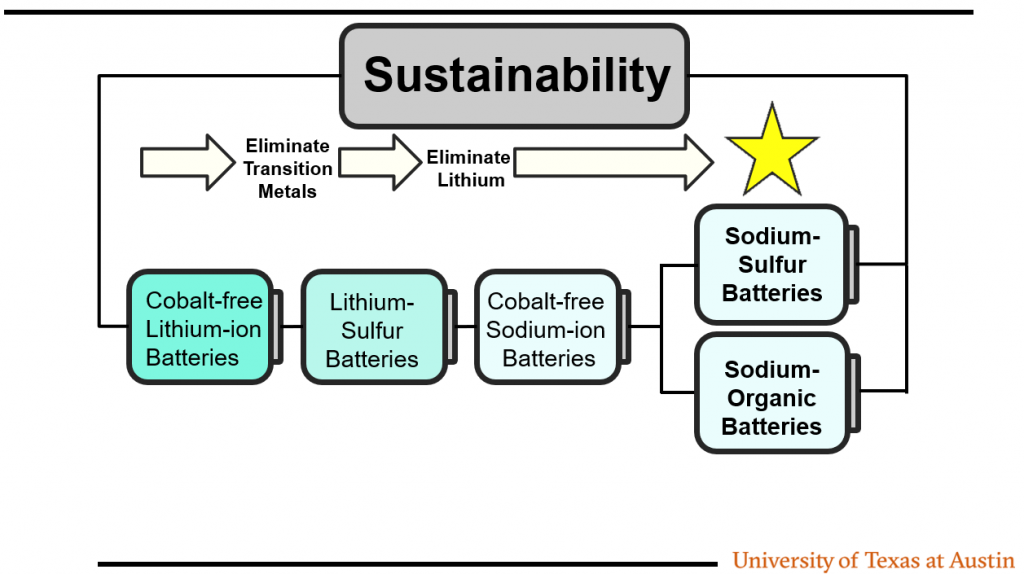
What is the single major challenge with sulfur batteries that is a showstopper?
Severe lithium-metal degradation caused by polysulfide shuttle.
This question concerns the cracking observed on the surface of the cathode materials which hinders its stability. Can this cracking not be beneficial for the functioning of the materials, as we observed with other materials with defects having outstanding performances?
Cracking in high-nickel cathodes is caused by severe surface reactivity with the electrolyte, formation of a thick CEI with harmful spinel-like and rock-salt phases, and consumption of active lithium in the cathode, so cracking is not good in high-nickel cathodes. See a paper my group published in Joule on October 8 for more information.
What property should we look for in electrolytes to make a stable high-energy-dense battery?
Less surface reactivity with both the anode and cathode.
In the Na-S systems, is the polysulfur electrolyte more like a liquid or a glass? You mentioned solid-solid transitions. Should we consider other glassy systems?
In the data I presented, the use of a newly developed electrolyte leads to the formation of a glassy phase on the cathode surface, which seems to alter the reaction pathway from solid-liquid-solid to a quasi soli-solid transition, resulting in better cycle life.
How to tackle the thermal runaway problem by excluding cobalt from NMC cathodes?
Potentially, through the development of a better electrolyte. See a paper my group published on October 8 in Joule for more information.
Could you please explain how a crack initiates because of Ni oxidation?
When Ni is oxidized to Ni4+, it tends to react aggressively with the electrolyte, causing a reduction of Ni4+ to Ni2+ and a removal of oxygen from the lattice, resulting in permanent cracks. See a paper my group published on October 8 in Joule for more information.
What is your opinion on other Li-alternative batteries, such as potassium or aluminum batteries?
Potassium batteries have good prospects.
Why doping Al and Mg?
Both Al and Mg are inert, and Mg-O and Al-O bonds are strong, so they help to provide a robust cathode surface that is less susceptible to the release of oxygen from the lattice.
How would you comment on the high electrolyte/sulfur ratio limiting energy density?
Adding too much electrolyte will also increase weight and reduce energy density.