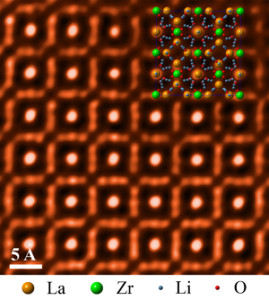
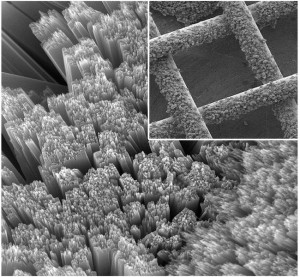
At left, a typical button battery; at right, a button battery coated with quantum tunneling composite (QTC).
Credit: Bryan Laulicht/MIT
We’ve heard a lot about innovation and improvements in the field of battery recently, but safety seems to have been put on the back-burner in lieu of creating a more powerful battery. This issue has now been addressed through funding from the National Institutes of Health in order to make technological breakthroughs in safety innovations for batteries.
According to the National Capital Poison Center, more than 3,500 people of all ages swallow button batteries every year in the United States. In order to combat the permanent injury that this could cause, researchers from MIT, Brigham and Women’s Hospital, and Massachusetts General Hospital have come together to create a coating that prevents batteries from conducing electricity after being swallowed – thereby causing no damage to the gastrointestinal tract.
Prior to this innovation, once a battery was swallowed, it would start to interact with the saliva and create an electric current. This current produces hydroxide, which causes damages to tissue. If not treated, this can cause serious injury within a few hours.