The National Academies of Sciences, Engineering, and Medicine study committee on Chemical Engineering: Challenges and Opportunities in the 21st Century seeks input from the chemical engineering community on the current state and future of the field. The ideas collected will assist the committee members as they outline a vision for chemical engineering research, education, and industrial innovation over the next 30 years.
Chemical Sciences Roundtable Webinar: Diversity, Equity, and Inclusion in Chemistry and Chemical Engineering
Posted on May 10, 2021 by Shannon Reed Register now for the virtual workshop on May 25-26, 2021
Register now for the virtual workshop on May 25-26, 2021
Keynote speakers
Freeman A. Hrabowski, III
President
University of Maryland, Baltimore County, U.S.
Geraldine (Geri) Richmond
Presidential Chair in Science and Professor of Chemistry
University of Oregon, U.S.
Date
Tuesday, May 25-Wednesday, May 26, 2021
Webinar is open to the public
 A research strategy for ocean carbon dioxide removal and sequestration
A research strategy for ocean carbon dioxide removal and sequestration
This summer, you may find yourself on the shore’s edge admiring the vastness and beauty of the ocean. There’s a lot going on in there! According to The National Academies of Sciences, Engineering, and Medicine, the ocean covers about 70% of the Earth’s surface and buffers a large fraction of anthropogenic CO2 emissions—removing roughly 55% of emitted CO2 naturally. BUT, it may be possible to enhance both the uptake and longer-term sequestration potential of these processes. The National Academies of Sciences, Engineering, and Medicine is looking to do just that and are currently soliciting nominations for individuals to serve on the Committee on A Research Strategy for Ocean Carbon Dioxide Removal and Sequestration. (more…)
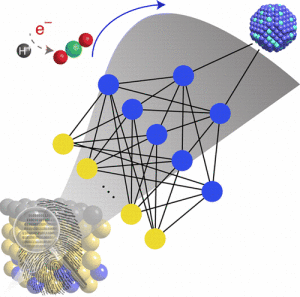 A novel development from Virginia Tech aims to “significantly accelerate materials discovery,” all while combating the pressing global warming issue.
A novel development from Virginia Tech aims to “significantly accelerate materials discovery,” all while combating the pressing global warming issue.
The new approach allows for efficient chemical conversions through a model that can predict novel alloy materials in a fast and accurate manner.
“This is the first example of learning from data in catalysis. We anticipate that this new research approach will have a huge impact in the future of materials design,” said Honglian Xin, lead author of the study.
Catalysts are hugely important in industry, with up to 90 percent of industrial chemicals being made from catalysts. These catalysts range from acids to nanoparticles, and even make up some enzymes in the human body.
Scientists have previously worked to improve catalysts through mixing metals with very precise atomic structures. While the results of these studies have led to metals with promising physical and chemical properties, the process has been costly and time consuming.
This from Virginia Tech:
That is why [the researchers] decided to use existing data to train computer algorithms to make predictions of new materials, a field called machine learning. The approach captures complex, nonlinear interactions of molecules on metal surfaces through artificial neural networks, thus allowing, “large scale exploration alloy materials space,” according to their article.
Lili Deligianni is a Research Scientist and Principal Investigator at IBM’s Thomas J. Watson Research Center. Her innovative work in chemical engineering has led to cutting-edge developments in chip technology and thin film solar cells. Lili has been with ECS for many years and currently serves as the Society’s Secretary.
Listen to the podcast and download this episode and others for free through the iTunes Store, SoundCloud, or our RSS Feed. You can also find us on Stitcher.
One brave man is distilling his own potent, yet drinkable, biofuel. Of course, there’s quite a bit of electrochemistry involved via this reflux still.
WARNING: Distilling alcohol is illegal in many places. (It can also be pretty dangerous for the novice distiller, so let’s leave this one to Hackett.)

Dr. Yossef Elabd, professor in the Artie McFerrin Department of Chemical Engineering at Texas A&M University, has developed two fuel cell vehicle platforms for both present day enhancements and future innovation.
Image: Texas A&M University
The Electrochemical Society’s Yossef A. Elabd is using electrochemical science to work toward global sustainability with his new advancements in fuel cell car technology.
Elabd, an active member of ECS’s Battery Division, has developed two fuel cell vehicle platforms for both present day enhancements and future innovation – focusing not only on the science, but also the environment.
“I just want to drive my car with water vapor coming out the back of it,” Elabd said.
With this new technology and initiatives such as the ECS Toyota Young Investigator Fellowship, Elabd’s statement may become an achievable reality for many people in the near future.
The idea of the fuel cell vehicle is every environmentalist’s dream, but the current issues deal with the sustainability of the vehicle. The current fuel cell car uses a proton exchange membrane (PEM) electrolyte for its platinum-based electrodes.
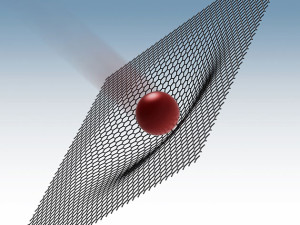
The ballistic test shows that graphene is excellent at both absorbing and spreading the energy of an impact.
Credit: Jae-Hwang Lee
We’ve been talking a lot about graphene – from its potential in energy storage to its ability to improve and revolutionize personal electronic devices, this material seems to be everywhere. Now, engineers out of the University of Massachusetts believe it could help save lives.
Engineers developed a mock-up of multilayered graphene body armor and tested it in a miniature shooting range. The results suggest that graphene may be able to absorb 10 times the amount of energy that its steel competitor can before failing.

ECS’s Shelley Minteer has developed a fuel cell that can convert jet fuel to electricity at room temperature without igniting the fuel.
Credit: Dan Hixson/University of Utah College of Engineering
The Electrochemical Society’s Shelley Minteer and her team of engineers at The University of Utah have developed the first room-temperature fuel cell that uses enzymes to help jet fuel produce electricity without need to ignite the fuel.
The new fuel cells will be able to be used to power portable electronics, off-grid power, and sensors.
The study was published in the American Chemical Society journal ACS Catalysis with Minteer as the senior author.
“The major advance in this research is the ability to use Jet Propellant-8 directly in a fuel cell without having to remove sulfur impurities or operate at very high temperature,” says Minteer. “This work shows that JP-8 and probably others can be used as fuels for low-temperature fuel cells with the right catalysts.”
The standard technique for converting jet fuel to electricity is both difficult, due to the sulfur content, and inefficient, with only 30 percent of the fuel converted to electricity under the best conditions.
This from The University of Utah:
To overcome these constraints, the Utah researchers used JP-8 in an enzymatic fuel cell, which uses JP-8 for fuel and enzymes as catalysts. Enzymes are proteins that can act as catalysts by speeding up chemical reactions. These fuel cells can operate at room temperature and can tolerate sulfur.
Minteer is a valued member of ECS and is on the editorial board of the Journal of The Electrochemical Society and ECS Electrochemistry Letters – along with being a past chair of the Physical and Analytical Electrochemistry Division. You can also read her published research in our Digital Library.
Make sure to sign up for our e-Alerts so you don’t miss the newest, cutting-edge research!

At left, a typical button battery; at right, a button battery coated with quantum tunneling composite (QTC).
Credit: Bryan Laulicht/MIT
We’ve heard a lot about innovation and improvements in the field of battery recently, but safety seems to have been put on the back-burner in lieu of creating a more powerful battery. This issue has now been addressed through funding from the National Institutes of Health in order to make technological breakthroughs in safety innovations for batteries.
According to the National Capital Poison Center, more than 3,500 people of all ages swallow button batteries every year in the United States. In order to combat the permanent injury that this could cause, researchers from MIT, Brigham and Women’s Hospital, and Massachusetts General Hospital have come together to create a coating that prevents batteries from conducing electricity after being swallowed – thereby causing no damage to the gastrointestinal tract.
Prior to this innovation, once a battery was swallowed, it would start to interact with the saliva and create an electric current. This current produces hydroxide, which causes damages to tissue. If not treated, this can cause serious injury within a few hours.



