
Image: Powin Energy
Powin Energy, a company focused on creating dynamic energy storage solutions, recently announced their plan to install a 30 kW/40 kW-hour battery system at the University of Washington’s Washington Clean Energy Testbeds. The testbed facility was developed by UW to scale-up, prototype, test, and validate new clean energy solutions. Powin Energy hopes to assist the researchers at the facility in their quest to develop clean energy innovation.
“We’re excited about this installation at the University of Washington because it will give our technology a more rigorous workout than most real-world installations that don’t approach the far ends of usage parameters,” Virgil Beaston, CTO of Powin Energy, said in a statement.
Venkat Subramanian, technical editor of the Journal of The Electrochemical Society and UW professor, discussed this energy storage opportunity, stating the he and his team could “use the Powin BESS to measure the performance of energy devices and algorithms when integrated into real and simulated system environments.”
Powin’s partnership with UW comes after the company’s development of its newly patented Battery Pack Operating system, which was designed to make its way into the utility-scale storage market. The company has already installed a 2MW/8MW-hour battery system in Irvine, CA.


 Twenty-sixteen marked the 25th anniversary of the commercialization of the lithium-ion battery. Since Sony’s move to commercialize the technology in 1991, the clunky electronics that were made possible by the development of the transistor have become sleek, portable devices that play an integral role in our daily lives – thanks in large part to the Li-ion battery.
Twenty-sixteen marked the 25th anniversary of the commercialization of the lithium-ion battery. Since Sony’s move to commercialize the technology in 1991, the clunky electronics that were made possible by the development of the transistor have become sleek, portable devices that play an integral role in our daily lives – thanks in large part to the Li-ion battery. New research shows another step forward in the goal of developing energy storage systems robust enough to store such intermittent sources as wind and solar on a large-scale.
New research shows another step forward in the goal of developing energy storage systems robust enough to store such intermittent sources as wind and solar on a large-scale.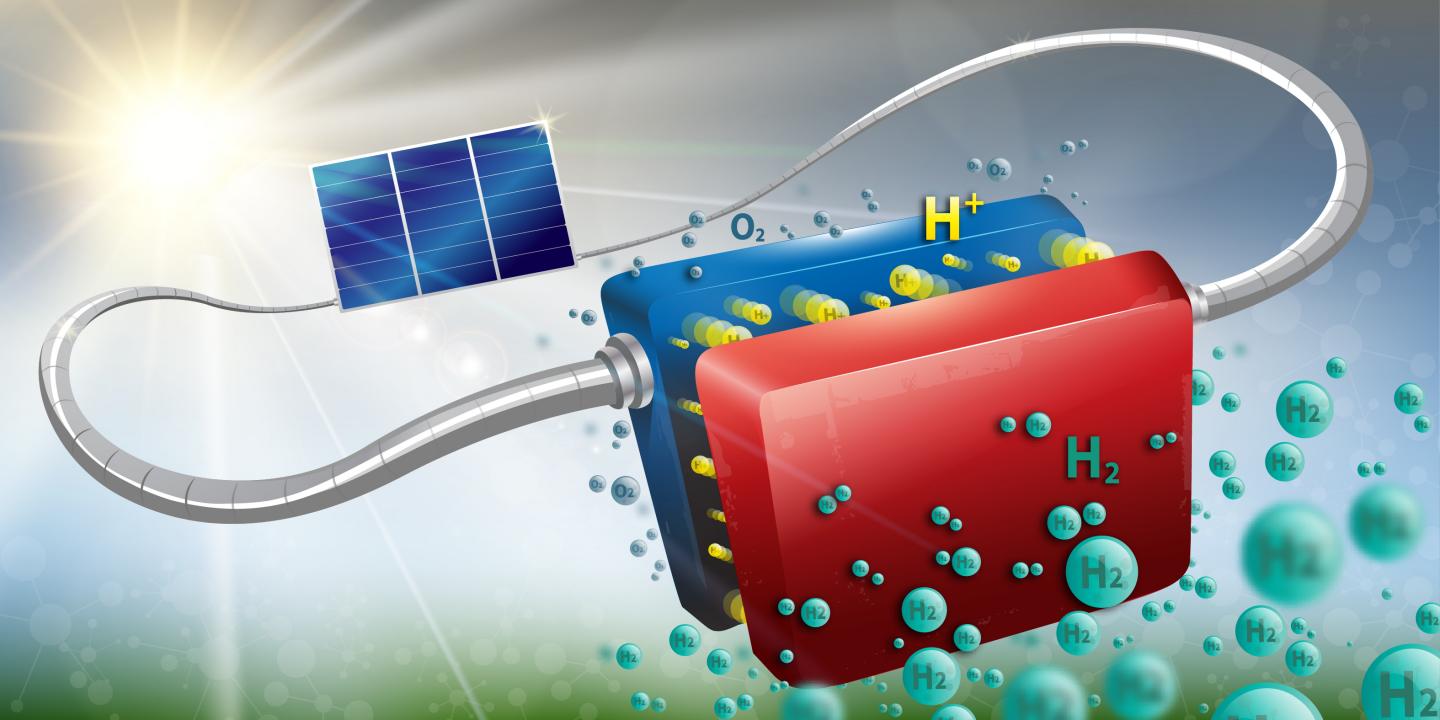
 The world’s next energy revolution is looming nearer.
The world’s next energy revolution is looming nearer.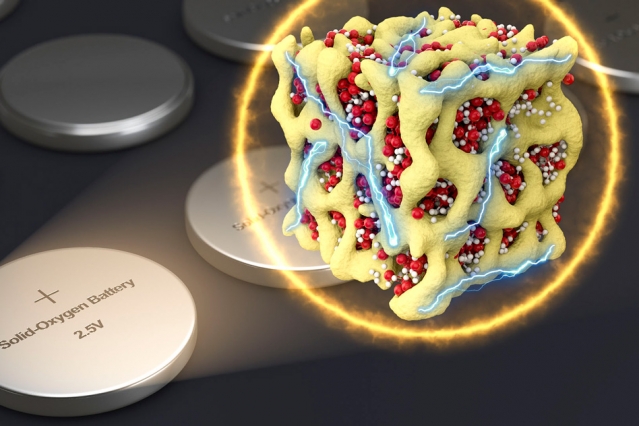
 The French research network on electrochemical energy storage (RS2E) – a public research organization focused on batteries and supercapacitors – has just launched the
The French research network on electrochemical energy storage (RS2E) – a public research organization focused on batteries and supercapacitors – has just launched the