By: Jonathan Coopersmith, Texas A&M University
 Imagine if you could gas up your GM car only at GM gas stations. Or if you had to find a gas station servicing cars made from 2005 to 2012 to fill up your 2011 vehicle. It would be inconvenient and frustrating, right? This is the problem electric vehicle owners face every day when trying to recharge their cars. The industry’s failure, so far, to create a universal charging system demonstrates why setting standards is so important – and so difficult.
Imagine if you could gas up your GM car only at GM gas stations. Or if you had to find a gas station servicing cars made from 2005 to 2012 to fill up your 2011 vehicle. It would be inconvenient and frustrating, right? This is the problem electric vehicle owners face every day when trying to recharge their cars. The industry’s failure, so far, to create a universal charging system demonstrates why setting standards is so important – and so difficult.
When done right, standards can both be invisible and make our lives immeasurably easier and simpler. Any brand of toaster can plug into any electric outlet. Pulling up to a gas station, you can be confident that the pump’s filler gun will fit into your car’s fuel tank opening. When there are competing standards, users become afraid of choosing an obsolete or “losing” technology.
Most standards, like electrical plugs, are so simple we don’t even really notice them. And yet the stakes are high: Poor standards won’t be widely adopted, defeating the purpose of standardization in the first place. Good standards, by contrast, will ensure compatibility among competing firms and evolve as technology advances.
My own research into the history of fax machines illustrates this well, and provides a useful analogy for today’s development of electric cars. In the 1960s and 1970s, two poor standards for faxing resulted in a small market filled with machines that could not communicate with each other. In 1980, however, a new standard sparked two decades of rapid growth grounded in compatible machines built by competing manufacturers who battled for a share of an increasing market. Consumers benefited from better fax machines that seamlessly worked with each other, vastly expanding their utility.
(more…)
 A new mathematical model may help researchers design new materials for use in high-power batteries. According to the research team, the model could benefit chemists and materials scientists who typically rely on a trial and error method when developing new materials for batteries and capacitors.
A new mathematical model may help researchers design new materials for use in high-power batteries. According to the research team, the model could benefit chemists and materials scientists who typically rely on a trial and error method when developing new materials for batteries and capacitors.

 One of the keys to developing a successful electric vehicle relies on energy storage technology. For an EV to be successful in the marketplace, it must be able to travel longer distances (i.e. over 300 miles on a single charge).
One of the keys to developing a successful electric vehicle relies on energy storage technology. For an EV to be successful in the marketplace, it must be able to travel longer distances (i.e. over 300 miles on a single charge). Imagine if you could gas up your GM car only at GM gas stations. Or if you had to find a gas station servicing cars made from 2005 to 2012 to fill up your 2011 vehicle. It would be inconvenient and frustrating, right? This is the problem electric vehicle owners face every day when trying to recharge their cars. The industry’s failure, so far, to create a universal charging system demonstrates why setting standards is so important – and so difficult.
Imagine if you could gas up your GM car only at GM gas stations. Or if you had to find a gas station servicing cars made from 2005 to 2012 to fill up your 2011 vehicle. It would be inconvenient and frustrating, right? This is the problem electric vehicle owners face every day when trying to recharge their cars. The industry’s failure, so far, to create a universal charging system demonstrates why setting standards is so important – and so difficult.
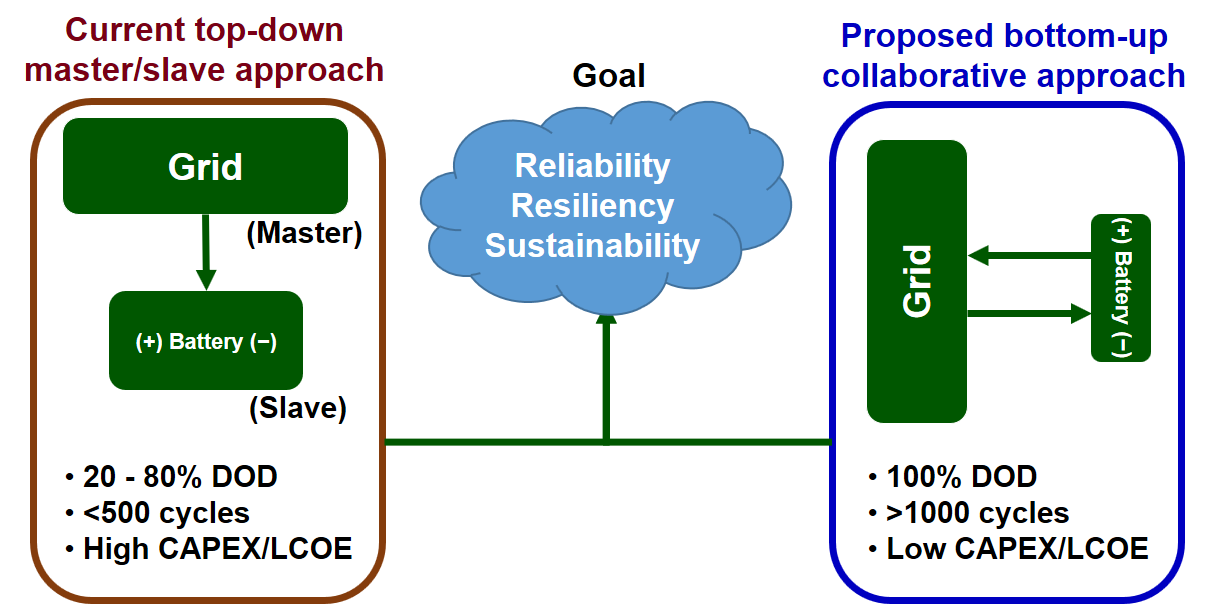
 Researchers from Oregon State university have developed the first battery that uses only hydronium ions as the charge carrier, which the team believes could yield promising results for the future of sustainable energy storage.
Researchers from Oregon State university have developed the first battery that uses only hydronium ions as the charge carrier, which the team believes could yield promising results for the future of sustainable energy storage.
 A new paper published in the Journal of The Electrochemical Society, “
A new paper published in the Journal of The Electrochemical Society, “ In an effort to develop an eco-friendly battery, researchers from Ulsan National Institute of Science and Technology (UNIST) have created a battery that can store and produce electricity by using seawater.
In an effort to develop an eco-friendly battery, researchers from Ulsan National Institute of Science and Technology (UNIST) have created a battery that can store and produce electricity by using seawater.