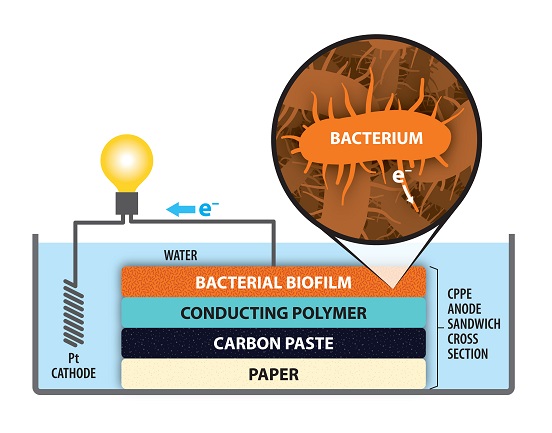
Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the 232nd ECS Meeting? Join us for a Ride-and Learn on Monday, October 2 from 12:00 pm to 2:00 pm in front of the main entrance of the Gaylord National Resort and Convention Center. This Ride-and-Learn is open to all ECS meeting attendees. First come, first serve.
Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the 232nd ECS Meeting? Join us for a Ride-and Learn on Monday, October 2 from 12:00 pm to 2:00 pm in front of the main entrance of the Gaylord National Resort and Convention Center. This Ride-and-Learn is open to all ECS meeting attendees. First come, first serve.
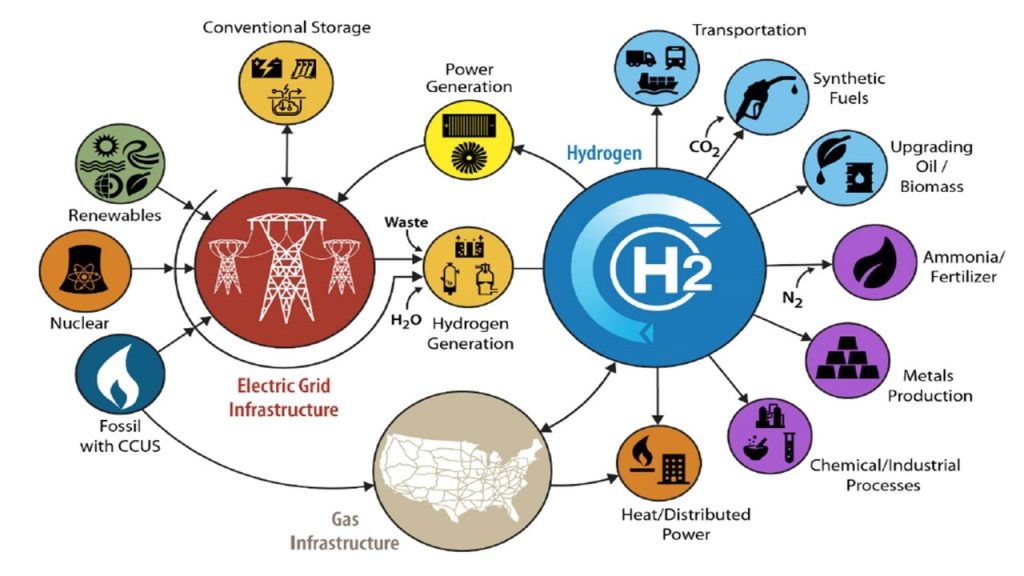
Fuel cell cars run on hydrogen fuel, use a fuel cell that converts hydrogen into the electricity that powers the car’s electric motor and emit only water from the tailpipe. For the first time ever, they are commercially available, have started hitting the streets and the hydrogen stations to fuel them are up and running in select U.S. regions.
This Ride-and-Learn is organized by the U.S. Department of Energy’s Fuel Cell Technologies Office (FCTO) in the Office of Energy Efficiency and Renewable Energy. FCTO has funded early-stage hydrogen and fuel cells research and development enabling a 60 percent reduction in fuel cell cost, a fourfold increase in fuel cell durability and an 80 percent cut in the cost of electrolyzers over the past decade. You can learn more about this exciting technology and the work FCTO funds to enable hydrogen and fuel cell technological breakthroughs at energy.gov/fuelcells.
Following the 232nd ECS Meeting, the third annual National Hydrogen and Fuel Cell Day will take place on October 8, 2017, aimed at raising awareness and celebrating advances in fuel cell and hydrogen technologies. The U.S. Department of Energy, Fuel Cell and Hydrogen and Energy Association , its members, industry organizations, and state and federal governments will be commemorating National Hydrogen and Fuel Cell day with a variety of activities and events across the country.
(more…)






 Using advanced computational methods, University of Wisconsin–Madison materials scientists have discovered new materials that could bring widespread commercial use of solid oxide fuel cells closer to reality.
Using advanced computational methods, University of Wisconsin–Madison materials scientists have discovered new materials that could bring widespread commercial use of solid oxide fuel cells closer to reality. Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the
Want to see Electrochemistry in Action and ride in one of the world’s first commercial fuel cell cars while at the