Image: CC0 Public Domain
Researchers at Los Alamos National Laboratory (LANL) are taking a closer look at fuel cell catalysts in hopes of finding a viable alternative to the expensive platinum and platinum-group metal catalysts currently used in fuel cell electrodes. Developments in this area could lead to more affordable next-generation polymer electrolyte fuel cells for vehicles.
The research, led by ECS fellow Piotr Zelenay, looks at the fuel cell catalysts at the atomic level, providing unique insight into the efficiency of non-precious metals for automotive and other applications.
“What makes this exploration especially important is that it enhances our understanding of exactly why these alternative catalysts are active,” Zelenay says. “We’ve been advancing the field, but without understanding the sources of activity; without the structural and functional insights, further progress was going to be very difficult.”
This from LANL:
Platinum aids in both the electrocatalytic oxidation of hydrogen fuel at the anode and electrocatalytic reduction of oxygen from air at the cathode, producing usable electricity. Finding a viable, low-cost PGM-free catalyst alternative is becoming more and more possible, but understanding exactly where and how catalysis is occurring in these new materials has been a long-standing challenge. This is true, Zelenay noted, especially in the fuel cell cathode, where a relatively slow oxygen reduction reaction, or ORR, takes place that requires significant ‘loading’ of platinum.


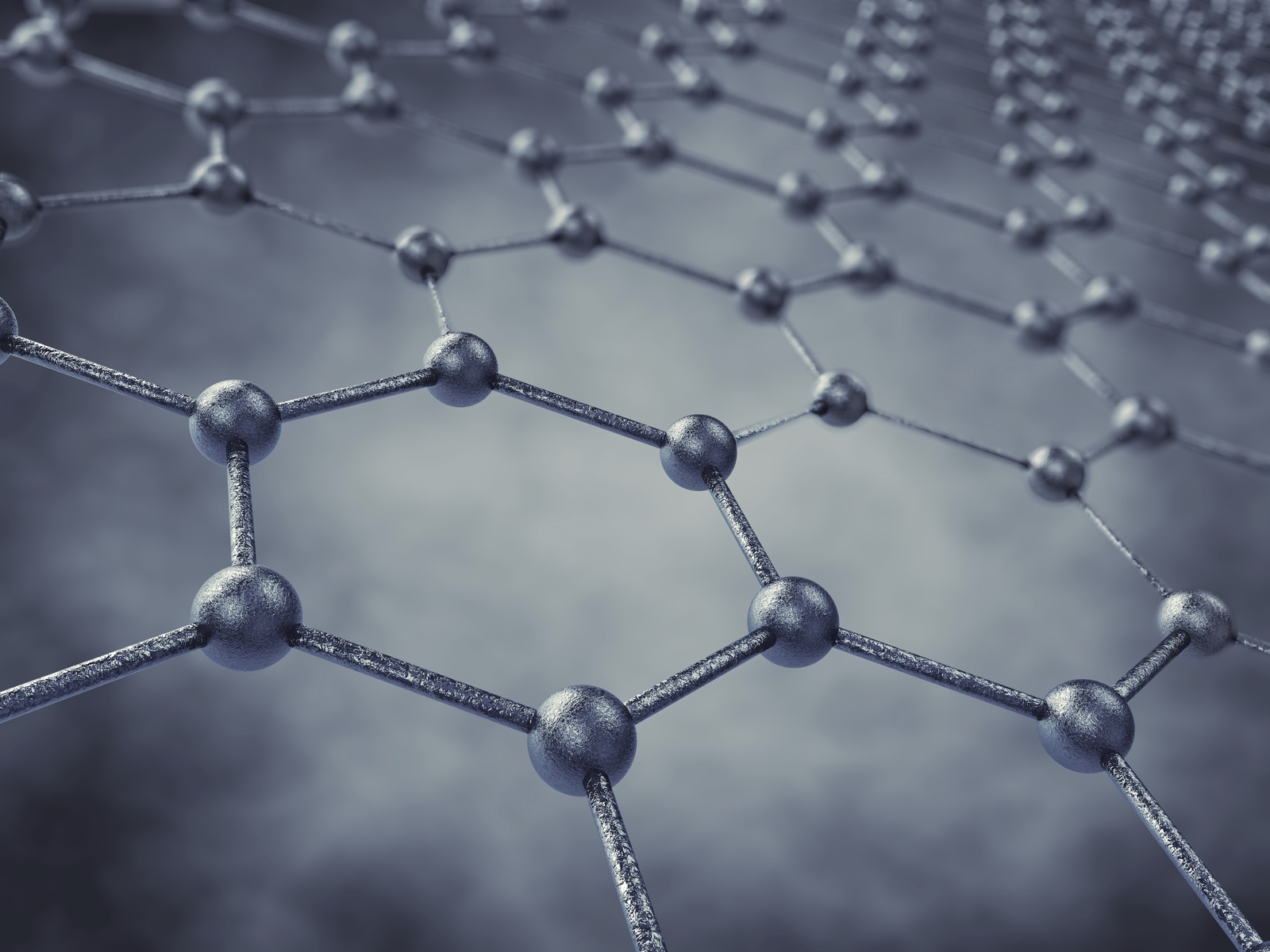 Scientists have created a durable catalyst for high-performance fuel cells by attaching single ruthenium atoms to graphene.
Scientists have created a durable catalyst for high-performance fuel cells by attaching single ruthenium atoms to graphene. The
The 
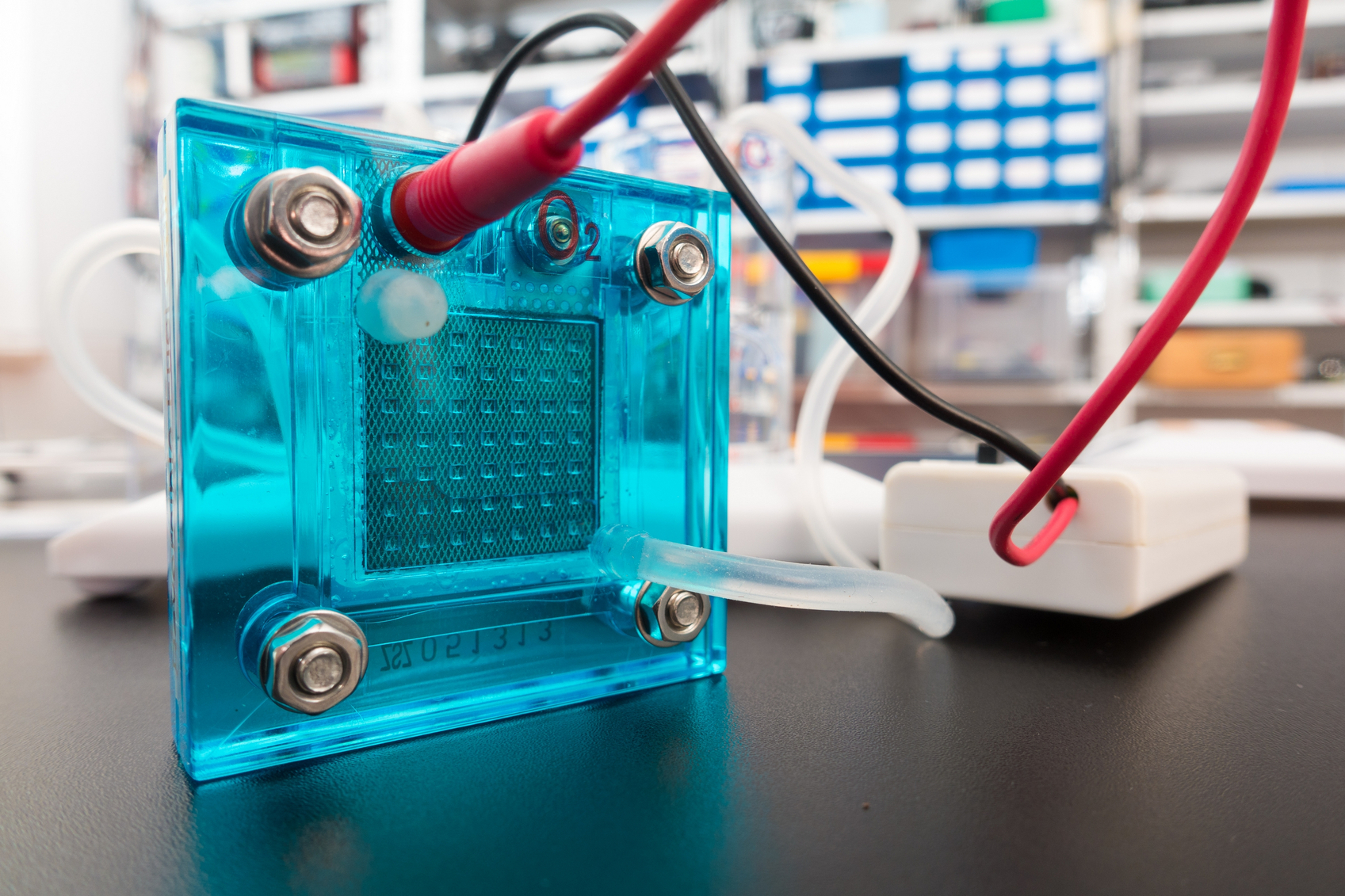 Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts.
Researchers from Purdue University are making headway on solving issues in electrolyzers and fuel cell development by gaining new insight into electrocatalysts. Sometimes the biggest advancements are the smallest in size.
Sometimes the biggest advancements are the smallest in size.