Websites of Note are gathered by Zoltan Nagy.
This is the latest Websites of Note, a regular feature in the ECS magazine Interface researched by Zoltan Nagy, a semi-retired electrochemist.
Lecture Notes in Electrochemistry/Electrochemical Engineering – M. Bazant, MIT
Detailed course material from MIT, including: equivalent circuit models, thermodynamics, reaction kinetics, transport phenomena, electrostatics, electrokinetics, porous media, and phase transformations.
Electroforming — a Unique Metal Fabrication Process – R. Parkinson, Nickel Development Institute
Electroforming plays an important role in our daily lives. We have contact with its results many times each day and it greatly enhances our lifestyle in a variety of ways. In addition, it is an extremely versatile process. For instance, it is used to produce micro components for the medical and electronics industries and huge components for the aircraft and aerospace industries. For many applications it has become indispensable.
Electrochemical Machining of Metal Plates – J.F. Cooper and M.C. Evans, Lawrence Livermore National Laboratory
Technical basis of electrochemical machining. Experimental basis of electrochemical machining. Theoretical basis of current distribution. Experimental tests and results (stationary cathode, advancing cathode, rotating cathode). Interpretations of results. Implementation of the process.
Electropolishing of Stainless Steels – Kosmač, Euro Inox
Electropolishing is a chemical surface finishing technique, by which metal is electrolytically removed, ion by ion, from the surface of a metal object. The primary objective is to minimize microroughness, thus dramatically reducing the risk of dirt or product residues adhering and improving the cleanability of surfaces. Electropolishing is also used for deburring, brightening, and passivating. The process exposes an undisturbed, metallurgically clean surface, eliminating thermal stress and surface roughening, and improving the corrosion resistance.
Dr. Nagy welcomes suggestions for entries; send them to nagyz@email.unc.edu.
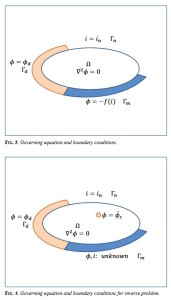 An article by Kenji Amaya, Naoki Yoneya, and Yuki Onishi published in the latest issue of Interface.
An article by Kenji Amaya, Naoki Yoneya, and Yuki Onishi published in the latest issue of Interface.