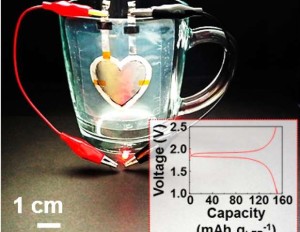
As electronics advances, the demand for high-performance batteries increases. The lithium-ion battery is currently leading the charge in powering portable electronic devices, but another lithium-based battery contender is on the horizon.
The lithium-air battery is one of the most promising research areas in current lithium-based battery technology. While researchers such as ECS’s K.M. Abraham have been on the Li-air beat since the late 90s, current research is looking to propel this technology with the hopes of commercializing it for practical use.
A new contender: Lithium-air batteries
Recently, Khalil Amine, IMLB chair; and Larry Curtiss, IMLB invited speaker, co-authored a paper detailing a lithium-air battery that could store up to five times more energy than today’s lithium-ion battery.
(MORE: Submit your abstract for IMLB today!)
This work brings society one step closer to the commercial use of lithium-air batteries. In previous works regarding Li-air, researchers continuously encountered the same phenomenon of the clogging of the pores of the electrode.