 Topic Close-up #1
Topic Close-up #1
Symposium D06: Quantum Dot Science and Technology 2
Deadline for Submitting Abstracts:
April 22, 2022
Submit today! (more…)
 Topic Close-up #1
Topic Close-up #1Symposium D06: Quantum Dot Science and Technology 2
Submit today! (more…)
 Researchers have found an explanation for why a certain class of quantum dots shines with such incredibly bright colors.
Researchers have found an explanation for why a certain class of quantum dots shines with such incredibly bright colors.
The nanocrystals in question contain caesium lead halide compounds arranged in a perovskite lattice structure. Three years ago, Maksym Kovalenko, a professor at ETH Zurich and the Swiss Federal Laboratories for Materials Science and Technology (Empa), succeeded in creating nanocrystals from the same semiconductor material.
“These tiny crystals have proved to be extremely bright and fast emitting light sources, brighter and faster than any other type of quantum dot studied so far,” says Kovalenko.
By varying the composition of the chemical elements and the size of the nanoparticles, Kovalenko also produced a variety of nanocrystals that light up with the colors of the entire visible spectrum. These quantum dots could be used as components for future light-emitting diodes (LEDs) and displays.
 In a new paper, researchers describe the underlying mechanisms involved in creating a widely used class of quantum dots that use cadmium and selenium compounds as their molecular precursors.
In a new paper, researchers describe the underlying mechanisms involved in creating a widely used class of quantum dots that use cadmium and selenium compounds as their molecular precursors.
For more than 30 years, researchers have been creating quantum dots—tiny, crystalline, nanoscale semiconductors with remarkable optical and electronic properties.
They’ve applied them to improve television sets, for example, to greatly enhance color. A host of other applications are in the works, involving integrated circuits, solar cells, computing, medical imaging, and inkjet printing, among others.
But quantum dot synthesis has occurred largely by trial and error, because little has been understood about how the chemicals involved in making quantum dots—some highly toxic—actually interact to form the resulting nanoparticles. The new research may change that, revealing more about the process of quantum dot formation.
Ironically, the team also discovered that, at one point during this process, the safer, more controllable compounds now employed decompose into the same highly toxic compounds that were used in initial quantum dot production 30 years ago.
 Tiny crystals called quantum dots are used in LCD TVs to enhance color and image quality. A few years ago, scientists discovered a new type of crystal called nanoplatelets.
Tiny crystals called quantum dots are used in LCD TVs to enhance color and image quality. A few years ago, scientists discovered a new type of crystal called nanoplatelets.
Like quantum dots, these two-dimensional structures are just a few nanometers in size, but have a more uniform flat, rectangular shape. They are extremely thin, often just the width of a few atomic layers, giving the platelets one of their most striking properties—their extremely pure color.
Now scientists have solved the mystery of out how these platelets form—and then created them in the lab using pyrite.
“We now know that there’s no magic involved in producing nanoplatelets, just science,” says David Norris, a materials engineering professor at ETH Zurich.
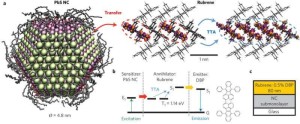 Quantum dots may be just the thing to take renewable energy technology to the next level.
Quantum dots may be just the thing to take renewable energy technology to the next level.
A team from MIT has recently developed a double film coating that has the ability to transform infrared light into visible light.
While that may not outwardly seem like a huge gain for the energy technology sector, the development has the potential to vastly improve efforts in renewable. Essentially, this research could help increase the amount of light a solar cell could capture. By capturing and using protons below their normal bandgap and thus converting the typically unused infrared light into use visible light, researchers could see efficiency levels of solar panels rise.
The researchers went about this development by placing two films on top of a plate of glass. The bottom film was comprised by using a type of quantum dot, while the top layer was made up of an organic molecule.
Andrea Guenzel, ECS Publications Specialist, recently spotted a CNN article on quantum dots and how they’re poised to change industry.
The technology behind Edison’s incandescent blub may be a thing of the past, but the warm, gentle glow that it produced may be making its way back into your living room.
But we’re not scrapping the advancements in LEDs and regressing to old technology to do this. Instead, we’re turning our attention to quantum dots—the tiny crystal-like particles that are 10,000 times smaller than the width of human hair.
And the dots’ applications do not end simply at bulbs. These tiny bursts of light are expected to impact displays, solar cells, and cancer imaging equipment as well.
Researchers from the University of Sydney have recently published their findings that quantum dots made of graphene can improve bio-imaging and LEDs.
The study was published in the journal Nanoscale, where the scientists detailed how activating graphene quantum dots produced a dot that would shine nearly five times bright than the conventional equivalent.
Essentially, the dots are nano-sized semiconductors, which are fluorescent due to their surface properties. However, this study introduces the utilization of graphene in the quantum dot, which produces an extra-bright dot that has the potential to help medicine.