 Topic Close-up #8
Topic Close-up #8
Symposium I01—Low Temperature Water Electrolysis (LT-WE) for H2 Production
Extended deadline for submitting abstracts:
December 16, 2022
Submit today!
 Topic Close-up #8
Topic Close-up #8Symposium I01—Low Temperature Water Electrolysis (LT-WE) for H2 Production
Submit today!
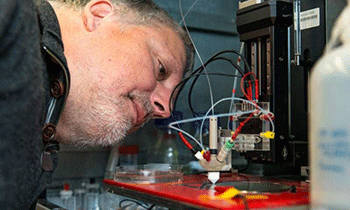
Together with his team, ECS member Wolfgang Schuhmann develops new electrodes, for the production of hydrogen.
Image: Ruhr Universitaet Bochum
New research out of Ruhr Universitaet Bochum is showing big gains for water electrolysis, with new efficiency levels double that of previous efforts.
By applying a layer of copper atoms in conventional platinum electrodes, researchers were able to desorption easier for the catalyst surface. This system then generated twice the amount of hydrogen than a platinum electrode without a copper layer.
This breakthrough could help water electrolysis gain a better reputation as a method for hydrogen production. Prior to this breakthrough, too much energy was lost in the process to prove it efficient. Now, the efficiency level has been doubled.
This from Ruhr Universitaet Bochum:
The researchers modified the properties of the platinum catalyst surface by applying a layer of copper atoms. With this additional layer, the system generated twice the amount of hydrogen than with a pure platinum electrode. But only if the researchers applied the copper layer directly under the top layer of the platinum atoms. The group observed another useful side effect: the copper layer extended the service life of the electrodes, for example by rendering them more corrosion-resistant.
“To date, hydrogen has been mainly obtained from fossil fuels, with large CO2 volumes being released in the process,” said Wolfgang Schuhmann, ECS member and lead author of the study. “If we succeeded in obtaining hydrogen by using electrolysis instead, it would be a huge step towards climate-friendly energy conversion. For this purpose, we could utilize surplus electricity, for example generated by wind power.”