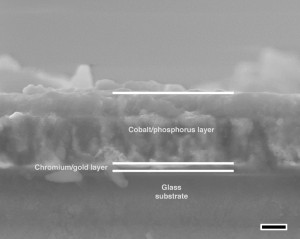
The lab fabricated the 500-nanometer films by anodizing a cobalt film electrodeposited on a substrate.
Image: Rice University
Researchers from Rice University have discovered an efficient, robust way of drawing hydrogen and oxygen from water.
The researchers have developed a new catalyst of a cobalt-based thin film, which pumps out hydrogen and oxygen to feed fuel cells.
This from Rice University:
The inexpensive, highly porous material invented by the Rice lab of chemist James Tour may have advantages as a catalyst for the production of hydrogen via water electrolysis. A single film far thinner than a hair can be used as both the anode and cathode in an electrolysis device.
The new cobalt film is more efficient at producing hydrogen than other materials and is much cheaper than commercial platinum catalysts.
“It is amazing that in water-splitting, the same material can make both hydrogen and oxygen,” Tour said. “Usually materials make one or the other, but not both.”
The researchers aim to make this an environmentally friendly source of hydrogen and oxygen by applying alternating current from renewable energy sources to cobalt-based electrolysis.
Read the full study in the journal Advanced Materials.
 Interested in water-splitting? Make sure to attend the 227th ECS Meeting this May in Chicago and catch the ECS Lecture with NREL’s John A. Turner entitled, “Hydrogen from Photoelectrochemical Water Splitting – What’s it gonna’ take?“.
Interested in water-splitting? Make sure to attend the 227th ECS Meeting this May in Chicago and catch the ECS Lecture with NREL’s John A. Turner entitled, “Hydrogen from Photoelectrochemical Water Splitting – What’s it gonna’ take?“.
And while you’re waiting for that meeting to roll around, listen to our podcast with Turner and check out some great papers on the topic in our Digital Library!

