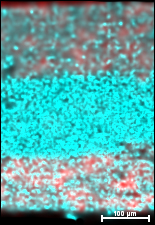
Engineers developed this one-material battery by sprinkling carbon (red) into each side of a new material (blue) that forms the electrolyte and both electrodes at the ends of the battery.
Source: Maryland NanoCenter
ECS student member Fudong Han and former member Chunsheng Wang have developed a novel solid state battery comprised of just one material that can both move and store electricity.
This new battery could prove to be revolutionary in the area of solid state batteries due to its incorporation of electrodes and electrolytes into a single material.
“Our battery is 600 microns thick, about the size of a dime, whereas conventional solid state batteries are thin films — forty times thinner. This means that more energy can be stored in our battery,” said Han, the first author of the paper and a graduate student in Wang’s group.
This from the University of Maryland:
The new material consists of a mix of sulfur, germanium, phosphorus and lithium. This compound is used as the ion-moving electrolyte. At each end, the scientists added carbon to this electrolyte to form electrodes that push the ions back and forth through the electrolyte as the battery charges and discharges. Like a little bit more sugar added at each end of a cookie-cream mixture, the carbon merely helps draw the electricity from side to side through the material.
An added benefit to this battery is the ease of production, consisting of only a powder compressed in a plastic and steel cylinder. However, the battery is not yet ready to be mass produced.
“We are still testing how many times it can change and discharge electricity to see if it is a real candidate for manufacturing,” said Han.
The battery will be able to charge and discharge smoothly due to its one material design.
ECS offers a variety of options for students to get involved, including a robust student chapter program. For more information, please click here.
PS: Both Han and Wang will be presenting at the 227th ECS Meeting. Check out the abstract for “In Situ Precipitated Organic Nanorod Electrodes for Sodium Ion Batteries” and register for the meeting today!

