 Lithium-air batteries are—in theory—an extremely attractive alternative for affordable, efficient energy storage for electric vehicles. However, as researchers explore this technology, they are met with many critical challenges. If researchers can overcome these challenges, there is a great likelihood that the lithium-air battery will surpass the energy density of today’s lithium-ion battery.
Lithium-air batteries are—in theory—an extremely attractive alternative for affordable, efficient energy storage for electric vehicles. However, as researchers explore this technology, they are met with many critical challenges. If researchers can overcome these challenges, there is a great likelihood that the lithium-air battery will surpass the energy density of today’s lithium-ion battery.
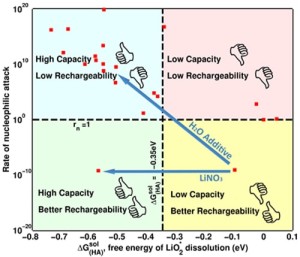
Researchers from Carnegie Mellon University and the University of California, Berkley feel like they may have part of the answer to this critical challenge, which could propel the practicality of the lithium-air battery. The team, which included researchers from Bryan McCloskey and Venkat Viswanathan‘s laboratories, has found a way to both increase the capacity while preserving the recharge ability of the lithium-air battery by blending different types within the battery’s electrolytes.
“The electrolytes used in batteries are just like Gatorade electrolytes,” says Venkat Viswanathan, assistant professor of mechanical engineering at Carnegie Mellon. “Every electrolyte has a solvent and a salt. So if you take Gatorade, the solvent would be water and the salt would be something like sodium chloride, for instance. However, in a lithium air battery, the solvent is dimethoxyethane and the salt is something like lithium hexafluorophosphate.”
Earlier this year, ECS members McCloskey and Viswanathan teamed up to improve the life-cycle of the lithium-air battery to five times of its current capacity. However, this was accomplished by adding a little bit of water to the battery’s electrolyte mixture, which prevents the battery from being recharged.
The new research, published in Proceedings of the National Academy of Science, addresses that issue.
“Our original idea was to add something to the solvent dimethoxyethane, and so we were initially exploring solvent additives. We found a fundamental problem with the solvent additives: the compromise to rechargeability. Then, McCloskey came up with the ingenious idea of changing the salt instead,” Viswanathan said. “Instead of using just one salt, we decided to blend salts together. We used two salts: lithium bis(trifluoromethane) sulfonimide and lithium nitrate.”
This new research is expected to yield great results for the big battery industry.
[Image: Carnegie Mellon University]
