The new structure has high mobility of Na+ ions and a robust framework.
Image: Nature Communications
With the demand for hand-held electronics at an all-time high, the costs of the materials used to make them are also rising. That includes materials used to make lithium batteries, which is a cause for concern when projecting the development of large-scale grid storage.
In order to find an alternative solution to the high material costs connected with lithium batteries, the researchers at the Australian Nuclear Science and Technology Organisation (ANSTO) and the Institute of Physics at the Chinese Academy of Science in Beijing have begun focusing their attention on sodium-ion batteries.
The science around sodium-ion batteries dates back to the 1980s, but the technology never took off due to resulting low energy densities and short life cycles.
However, the new research looks to combat those issues by improving the properties of a class of electrode materials by manipulating their electron structure in the sodium-ion battery.
“The connection between sodium-ion ordering and charge distribution in the framework was established a long time ago. However, so far most of the research into breaking the connection was based on combining several electrochemically active transition metals, such as iron, manganese, cobalt and nickel that was either ineffective or had side effects,” said Max Avdeev, one of the corresponding authors of the recently published paper.
This from ANSTO:
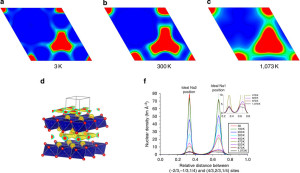
To probe the effect of inactive ions, a compound, Na0.6(Cr0.6TiO4)O2 was synthesised and thoroughly investigated electrochemically and by neutron scattering at the Chinese Academy of Sciences and ANSTO, respectively. It was confirmed by neutron diffraction experiments on the Echidna instrument at OPAL which was used to study Na0.6(Cr0.6TiO4)O2, in a wide range of temperatures and sodium content. Understanding all the conditions that led to sodium ordering was critical to design the material with desired properties.
Head over to the Digital Library to read more about sodium-ion batteries.

