 A new form of carbon that has unprecedented strength and magnetism properties is making its mark in the world of materials science.
A new form of carbon that has unprecedented strength and magnetism properties is making its mark in the world of materials science.
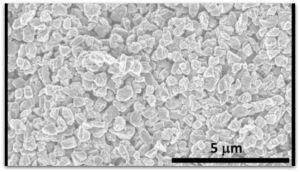
Researchers from North Carolina State University have recently developed a new phase of carbon called Q-carbon—an extraordinarily strong material that differs from carbon’s other two solid forms.
The first solid phase of carbon is graphite. Graphite is composed by lining up carbon atoms to form thin sheets, which results in a thin and flaky material. The other phase of carbon, diamond, occurs when carbon atoms form a rigid crystal lattice.
Third Phase of Carbon
“We’ve now created a third solid phase of carbon,” says Jay Narayan, lead author of the research. “The only place it may be found in the natural world would be possibly in the core of some planets.”
Q-carbon differs from both existing phases of carbon, with unique characteristics that researchers did not even think were possible prior to its development, such as its magnetic and glowing qualities. To fully understand its novel qualities, it’s essential to understand how Q-carbon was developed.
This from North Carolina State University:
Researchers start with a substrate, such as such as sapphire, glass or a plastic polymer. The substrate is then coated with amorphous carbon – elemental carbon that, unlike graphite or diamond, does not have a regular, well-defined crystalline structure. The carbon is then hit with a single laser pulse lasting approximately 200 nanoseconds. During this pulse, the temperature of the carbon is raised to 4,000 Kelvin (or around 3,727 degrees Celsius) and then rapidly cooled. This operation takes place at one atmosphere – the same pressure as the surrounding air.
Potential Applications
The end product is a film of Q-carbon between 20 and 500 nanometers thick. One possible application for Q-carbon could be super-thin, durable screen displays. However, researchers are still working to understand the properties to Q-carbon and all its potential.
“We can make Q-carbon films, and we’re learning its properties, but we are still in the early stages of understanding how to manipulate it,” Narayan says. “We know a lot about diamond, so we can make diamond nanodots. We don’t yet know how to make Q-carbon nanodots or microneedles. That’s something we’re working on.”
[Image: Journal of Applied Physics]
