Image: Zosia Rostomian/Berkeley Lab
Scientists can now directly probe hard-to-see layers of chemistry due to the development of an X-ray toolkit out of Lawrence Berkeley National Laboratory.
The research team behind the initiative believes that their development could provide insight about battery performance and corrosion. Additionally, it could give insight into a variety of chemical reactions, including biological and environmental processes.
The from LBNL:
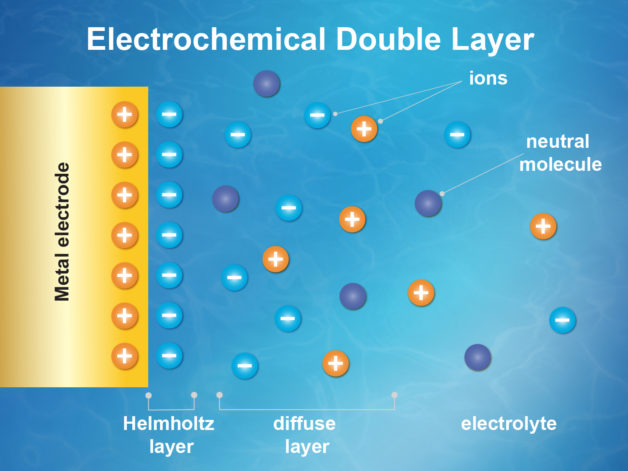
In a first-of-its-kind experiment at Berkeley Lab’s Advanced Light Source, an X-ray source known as a synchrotron, researchers demonstrated this new, direct way to study the inner workings of an activity center in chemistry known as an “electrochemical double layer” that forms where liquids meets solids—where battery fluid (the electrolyte) meets an electrode, for example (batteries have two electrodes: an anode and a cathode).
In a battery, changes in electrical potential can be seen in the electrochemical double layer.
“To be able to directly probe any attribute of the double layer is a significant advancement,” says Ethan Crumlin, co-author of the study and previously published ECS author. “Essentially, we now have a direct map, showing how potential within the double layer change based on adjustments to the electrode charge and electrolyte concentration. Independent of a model, we can directly see this—it’s literally a picture of the system at that time. This will help us with guidance of theoretical models as well as materials design and development of improved electrochemical, environmental, biological and chemical systems.”
ECS Fellow Hubert Gasteiger commented on the work, saying, “This work is already quite applicable to real problems. No one has been able to look into this roughly 10-nanometer-thin region of the electrochemical double layer before. This is one of the first papers where you have a probe of the potential distribution here. Using this too to validate double-layer models I think would give us insight into many electrochemical systems that are of industrial relevance.”

