 The cleaning of industrial wastewater is a persistent issue across the globe. If left untreated, these harmful waters could enter open watercourses, dispersing contaminants such as mercury and lead. Not only is this an immediate health risk, but it also threatens the entire ecosystem.
The cleaning of industrial wastewater is a persistent issue across the globe. If left untreated, these harmful waters could enter open watercourses, dispersing contaminants such as mercury and lead. Not only is this an immediate health risk, but it also threatens the entire ecosystem.
Modern wastewater treatment plants have been able to treat the water, but have not been very environmentally conscious. The typical plant produces CO2 by burning fossil fuels for power and the general decomposition of the materials in the wastewater. Not to mention, these things require a lot of power. About 12 trillion gallons of wastewater gets treated each year in the United States along, consuming an alarmingly high 3 percent of the nation’s energy grid.
Researchers have already produced power from pee and made poop potable; so why not develop a new type of wastewater treatment device that significantly lessens the severity of CO2 emissions and simultaneously captures greenhouse gases?
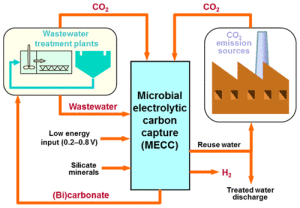
That’s exactly what researchers from the University of Colorado at Boulder have done. It’s called Microbial Electrolytic Carbon Capture (MECC), and it focuses on an electrochemical reaction the both cleans wastewater and absorbs the majority of CO2 produced.
“The idea is that the microbes can really get into dirty situations and deal with waste and conditions that any other electrochemical device won’t be able tolerate,” said Gemme Regurera, an associate professor in microbiology and molecular genetics and winner of our “Science for Solving Society’s Problems Challenge“—a project focused on addressing critical technology gaps in water, sanitation, and hygiene.
Additionally, this environmentally-friendly process produces clean, renewable energy.
“This energy-positive, carbon-negative method could potentially contain huge benefits for a number of emission-heavy industries,” said Zhiyong Jason Ren, former member of ECS and senior author of the new study. (Read some of Ren’s past publications.)
This from University of Colorado at Boulder:
Existing carbon capture technologies are energy-intensive and often entail costly transportation and storage procedures. MECC uses the natural conductivity of saline wastewater to facilitate an electrochemical reaction that is designed to absorb CO2 from both the water and the air. The process transforms CO2 into stable mineral carbonates and bicarbonates that can be used as raw materials by the construction industry, used as a chemical buffer in the wastewater treatment cycle itself or used to counter acidity downstream from the process such as in the ocean.
This process may also be beneficial to fuel cell technology. The electrochemical reaction produces hydrogen gas, which could be stored and later harnessed.
“This treatment system generates alkalinity through electrochemical means and we could potentially use that to help offset the effects of ocean acidification,” said Greg Rau, a senior researcher and co-author of the study. “This is one of several environmentally-friendly things this technology does.”
[Image: Environmental Science & Technology]
